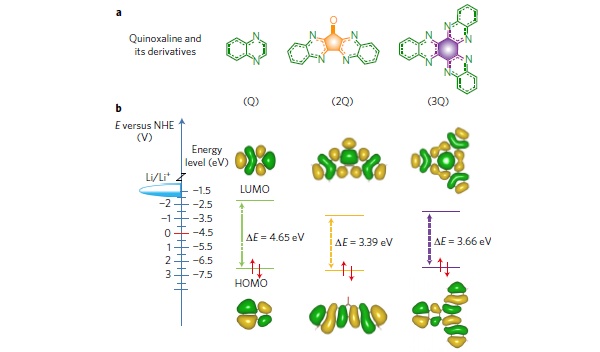
Even though organic molecules with well-designed functional groups can be programmed to have high electron density per unit mass, their poor electrical conductivity and low cycle stability limit their applications in batteries. A team led by Prof Loh Kian Ping report the facile synthesis of π-conjugated quinoxaline-based heteroaromatic molecules (3Q) by condensation of cyclic carbonyl molecules with o-phenylenediamine. 3Q features a number of electron-deficient pyrazine sites, where multiple redox reactions take place, which was probed using in-situ 15N and 13C solid state Nuclear Magnetic Resonance. When hybridized with graphene and coupled with an ether-based electrolyte, an organic cathode based on 3Q molecules displays a discharge capacity of 395 mAh g−1 at 400 mA g−1 (1C) in the voltage range of 1.2–3.9 V and a nearly 70% capacity retention after 10,000 cycles at 8 A g−1. This work is important in demonstrating that heteroaromatic molecules with multiple redox sites are promising in developing high-energy-density, long-cycle-life organic rechargeable batteries.
