The research team, led by Assistant Professor ZHU Ru-Yi from the Department of Chemistry at NUS have found a new use for deoxyribonucleic acid (DNA), not just as genetic material, but as a tool for more efficient production of medicinal compounds. Certain parts of DNA, called phosphates, can act like tiny “hands” that guide chemical reactions to selectively produce the desired mirror-image version of a compound.
They discovered that certain phosphate groups in DNA can attract and guide positively charged reactants during a chemical reaction. This is similar to a magnet gently pulling a metal bead into the correct orientation. This “ion-pairing” effect holds the reactants close and in a particular orientation, steering the reaction in a specific way to produce only one mirror-image product. The team demonstrated this effect across several different types of chemical reactions.
The research was published in the scientific journal Nature Catalysis.
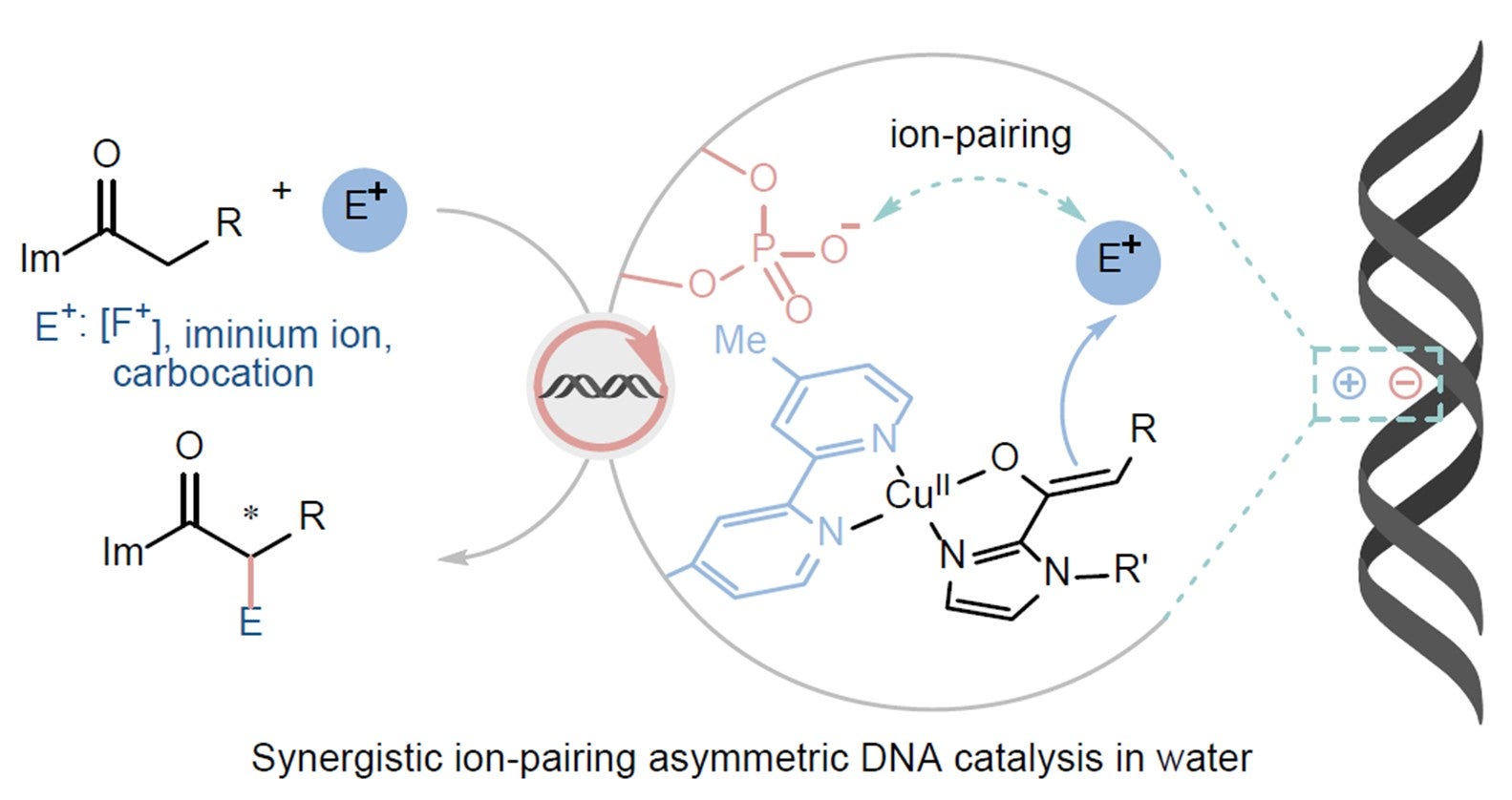
Ionic interactions involving DNA phosphates are ubiquitous and essential across all kingdoms of life. In this study, their catalytic potential has been demonstrated in a variety of asymmetric transformations through ion-pairing interactions in water. DNA’s negatively charged phosphates can pair with positively charged reagents, allowing DNA to act as a chiral catalyst in water. [Credit: Nature Catalysis]
Assistant Professor Zhu said, “Nature never uses DNA phosphates as catalysts, but we have shown that if designed properly, they can act like artificial enzymes.”
“Beyond being a conceptual breakthrough, this method could make chemical manufacturing more sustainable and environmentally friendly, especially for producing complex, high-value molecules used in pharmaceutical products,” added Assistant Professor Zhu. Read the full article here.
