
Associate Professor WU Jie and his research group from the Department of Chemistry at NUS, together with Professor ZHAO Yu, also from the same department, introduced a groundbreaking solution. They have developed a straightforward method to convert common chemicals like carboxylic acids, alcohols, and alkanes directly into valuable alkenes.
This work was conducted in collaboration with Professor MA Jun-an from Tianjin University, China. Their new method combines two known chemical reactions—photocatalytic radical addition and Norrish type II reaction—into a single, seamless process powered by light. The researchers used an easily accessible and reusable chemical called vinyl ketone as the “olefination reagent” to help create alkenes. By fine-tuning the reaction parameters of the vinyl ketone, they were able to enhance the reaction while minimising unwanted side reactions. These findings were published in the journal Nature Chemistry.
Prof Wu said, ” As detailed in the research paper, this method provides an easy way to create useful alkenes from many different starting materials. In the future, we plan to extend this method to work with even more types of feedstock chemicals and to explore the control of alkene geometry. We believe our work will become a valuable tool for research in the pharmaceutical and agricultural fields.” Read the full article here.
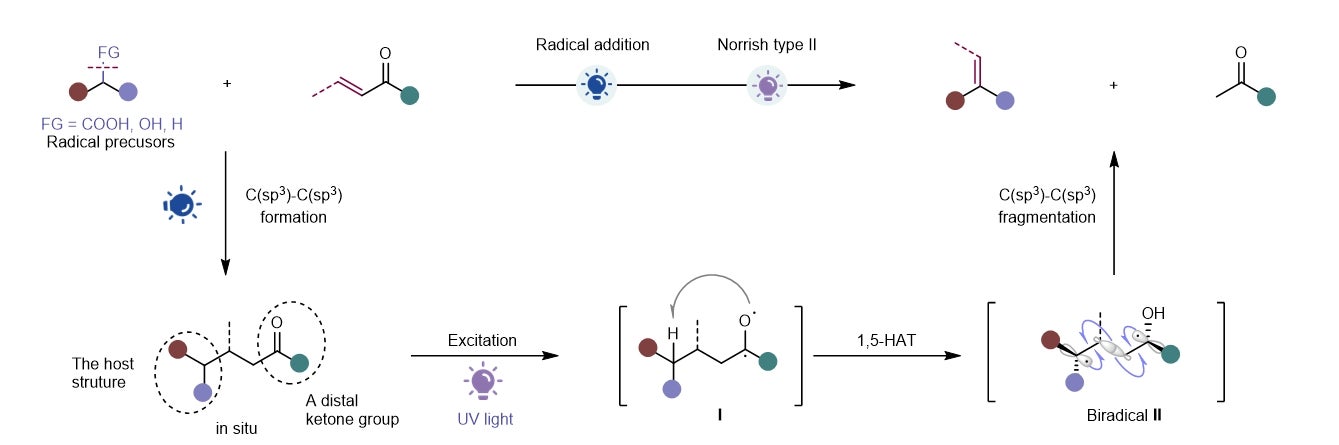
Schematic showing the development of an integrated photochemical strategy that introduce alkene moieties from a vast collection of diverse substrates. It involves two key steps, the radical addition and Norrish type II reaction. [Credit: Nature Chemistry]
