
The research team led by Associate Professor KOH Ming Joo and Professor ZHAO Yu from the NUS Department of Chemistry have developed an electrochemical reaction manifold that promotes efficient nitrogen atom insertion into saturated carbocycles to access either functionalised quinolines or N-alkylated saturated N heterocycles, both of which are privileged scaffolds in synthetic chemistry and pharmaceutical science. The research breakthrough was published in the scientific journal Nature Synthesis.
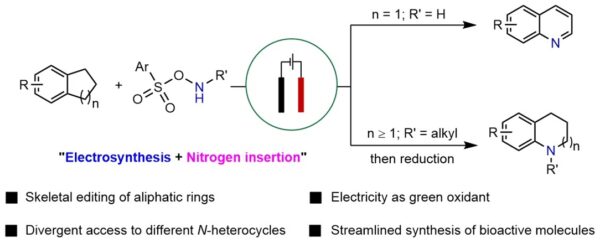
Divergent electrochemical nitrogen atom insertion into saturated carbocycles to access diverse valuable N heterocycles. [Credit: Nature Synthesis]
Assoc Prof Koh said, “We wanted to design a greener and controllable way of transforming saturated carbocycles into different classes of N heterocycles. This would greatly facilitate the sustainable synthesis of valuable N-heterocyclic bioactive compounds for applications such as drug discovery.”
“By strategically modulating the oxidation or reduction of the key cyclic imine intermediate, a broad array of substituted quinolines and N-alkylated N heterocycles bearing synthetically valuable functional groups can be efficiently accessed. We believe this methodology offers a versatile and robust platform for the modification and synthesis of bioactive scaffolds,” added Assoc Prof Koh.
Studies are ongoing to employ the methodology to synthesize other types of heterocyclic bioactive compounds. Read the full article here.
