A research team led by Assistant Professor KOH Ming Joo, from the Department of Chemistry, National University of Singapore has developed an effective method to access enantioenriched drug-like compounds through multicomponent olefin cross-coupling using chiral nickel-based catalysts. This new strategy leverages widely available nickel catalysts containing hindered N-heterocyclic carbene (NHC) ligands, to merge olefins with an organotriflate and a metal alkoxide as hydride donor. Replacing the metal alkoxide with an organometallic reagent enables installation of two different carbogenic groups. These multicomponent reactions provide a streamlined pathway towards chiral molecules bearing enantioenriched carbon- or heteroatom-substituted tertiary or quaternary stereogenic centres. This is a collaboration with Professor Shi-Liang SHI, from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. The findings were published in Nature Catalysis.
Prof Koh said, “Through selective carbofunctionalizations, we can even access opposite enantiomers of a chiral molecule by using a single chiral catalyst antipode. This is difficult to achieve using alternative systems, demonstrating a unique advantage of our catalytic regime. We can now utilise this new protocol as a general platform to produce valuable chiral molecules with high stereochemical purity.”
“We believe this methodology will significantly enrich the toolbox of asymmetric catalysis to facilitate countless applications in stereoselective natural product synthesis and drug discovery,” added Prof Koh.
The research team is developing new chiral NHC-nickel catalysts to promote olefin cross-coupling transformations that can potentially address other unresolved challenges in organic synthesis. Read the full article here.
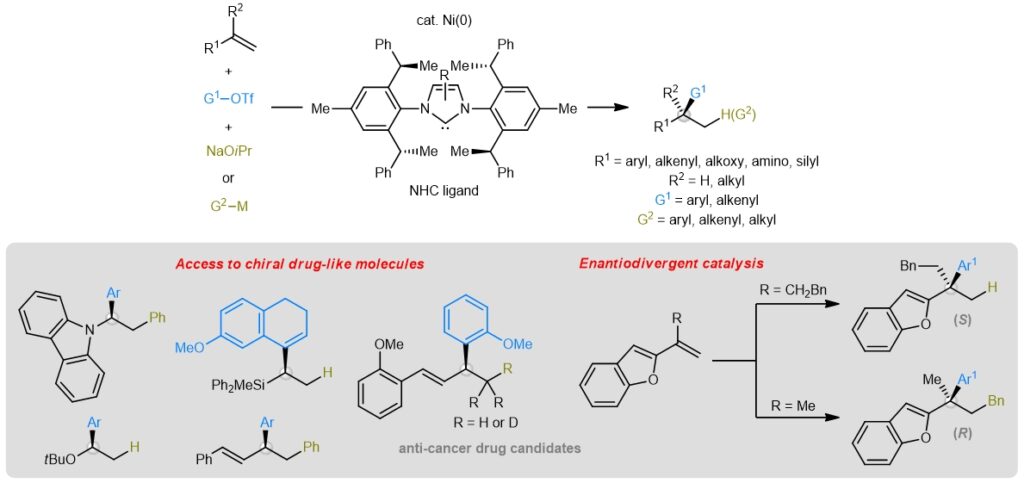
Figure 1: Schematic showing the design of a chiral nickel-catalysed manifold that transforms cheap and abundant olefins to high-value drug-like building blocks. [Credit: Nature Catalysis]
