A research team led by Assistant Professor ZHU Ru-Yi from the Department of Chemistry at NUS developed a purely chemical, post-synthetic approach that temporarily “cages” RNA by modifying its 2′-OH sites with carefully tuned disulfide-containing acyl groups. These modifications can temporarily block the RNA from carrying out its natural biological activity until intracellular glutathione (GSH), a common reducing agent, acts as the “key” by triggering a redox reaction that removes the acyl groups. By adjusting the chemical structure and properties of these acyl groups, the researchers can achieve fast, efficient, and controllable RNA activation for diverse RNA types, from short synthetic strands to long messenger RNAs (mRNAs).
The findings were published in the journal Angewandte Chemie International Edition.
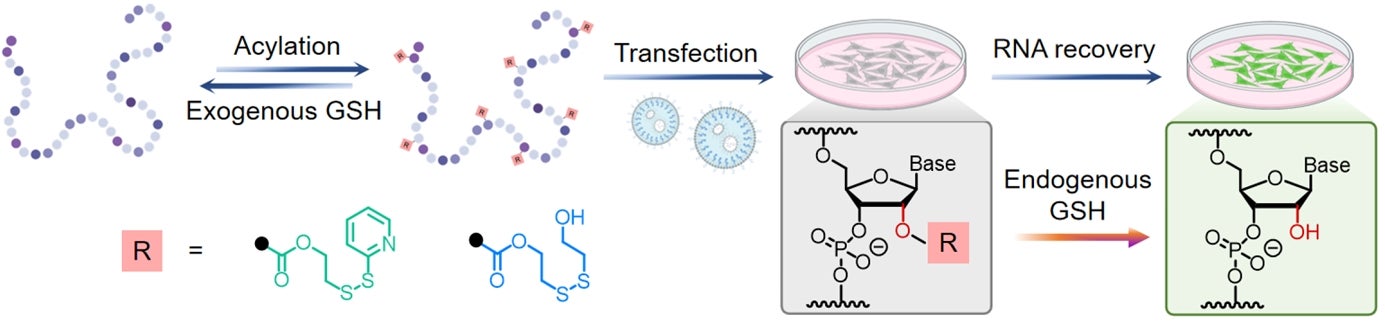
This work presents a redox-responsive strategy for precise RNA control via post-synthetic 2′-OH acylation. These introduced disulfide-containing acyl groups can be selectively cleaved upon exposure to both exogenous and endogenous glutathione (GSH), enabling traceless RNA release and functional recovery. This approach offers a versatile tool for modifying and manipulating RNAs of diverse lengths or origins. [Credit: Angewandte Chemie International Edition]
Assistant Professor Zhu said, “Our approach provides a universal method to modulate RNA activity with spatial and temporal control, without relying on enzymes or light. This is the first example such responsive mRNA activation has been shown to work in both test tube and live-cell environments.”
Through a series of systematic optimisations, the team established three distinct chemical methods to acylate RNA post-synthetically. These methods enable reversible blocking of the RNA function and can be triggered to release RNA by either natural or externally supplied GSH. “The simplicity and broad compatibility of our redox-responsive acylation system make it accessible to a wide range of researchers working with RNA,” added Assistant Professor Zhu. Read the full article here.
