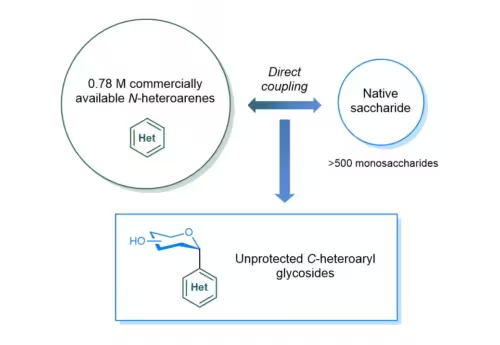
The research team led by Associate Professor KOH Ming Joo from the NUS Department of Chemistry and Professor CHAN Chun Yong Eric from the NUS Department of Pharmacy and Pharmaceutical Sciences have developed a “capping-and-coupling” strategy to transform naturally occurring (native) sugars directly into compounds known as C-heteroaryl glycosides. This makes it easier to produce such molecules that are valuable for drug and vaccine development. The research breakthrough was published in the scientific journal Nature Synthesis.
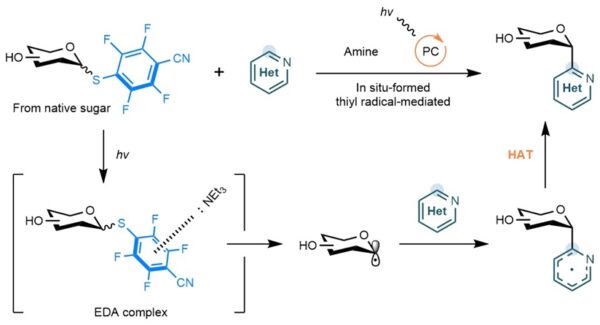
Schematic illustration of the one-step activation of native sugars followed by photocatalytic coupling with N-heteroarenes to deliver C-heteroaryl glycosides. The sugar compound is first selectively activated at the anomeric (C1) position, then exposed to an amine, a photocatalyst and visible light, allowing it to form a new carbon–carbon bond with a nitrogen-containing aromatic molecule. This streamlined process avoids many of the usually required protective steps. [Credit: Nature Synthesis]
Associate Professor Koh said, “The most appealing way to make C-heteroaryl glycosides and create new functional molecules is to merge naturally occurring native saccharides with N-heteroarenes, both of which are prevalent in nature, through direct carbon-carbon bond formation.” Read the full article here.
