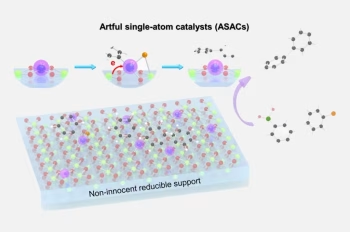
A research team led by Associate Professor LU Jiong, from the Department of Chemistry, NUS developed an “anchoring-borrowing” strategy to create a new class of artful single-atom catalysts (ASACs). The key idea behind this innovation involves anchoring single metal atoms onto specific sites of metal oxide surfaces. These surfaces can “borrow” oxygen atoms from their surroundings to act as anchor points, while using the metal oxide as an electron reservoir. This unique design allows the structure to adapt and change in a way that avoids the high demand for complex electronic changes in the metal itself, which is a common challenge in traditional cross-coupling reactions. This work is a collaborative effort with Associate Professor WU Jie from NUS Chemistry, Associate Professor WANG Yang-Gang from Southern University of Science and Technology, China, Assistant Professor WU Dongshuang from Nanyang Technological University, Singapore, and Assistant Professor HAI Xiao from Peking University, China. This research was published in the journal Nature Communications.
Prof Lu said, “The new concept of heterogeneous ASACs provides a much greener way to tackle the long-standing challenge of oxidative addition in cross-coupling reactions. This strategy goes beyond the limitations of traditional homogeneous and heterogeneous catalysts, and holds great potential for large-scale, sustainable production of fine chemicals and pharmaceuticals.” ‘Looking ahead, we plan to extend this approach to a wider range of metals that can be used in cross-coupling reactions. By adjusting the types and combinations of single atoms and support materials, we could enhance the performance of more abundant, non-precious metals in these reactions,” added Prof Lu.

(a) Rational design of ASAC for cross-coupling reactions, where M1 represents the foreign single metal atom introduced onto the reducible carriers. (b) Using a single atom of Pd1 anchored on the material CeO2 as a representative example, this panel illustrates the dynamic structural and valence state evolution of the Pd1 ASAC. These changes help it avoid the usual challenge in cross-coupling reactions, which is the energy barrier associated with oxidative addition. This contrasts with traditional homogeneous catalyst systems, where the reaction rate is largely limited by this step. [Credit: Nature Communications]
