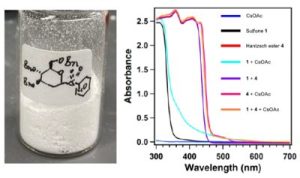
A research team led by Assistant Professor KOH Ming Joo has developed a new strategy to generate therapeutically relevant C-glycosides and S-glycosides through a catalyst- and transition metal-free approach under visible light illumination at ambient temperature. The team discovered that these bench-stable sulfones are also redox-active. By using visible (blue) light illumination and a Hantzsch ester-base complex, the researchers are able to activate the sulfones to produce chemically reactive glycosyl radicals. These radicals react readily with various electrophiles (see Figure 1). With this method, they are able to obtain a wider range of valuable C-alkyl, C-alkenyl, C-alkynyl, C-heteroaryl and S-linked glycosides in an efficient and highly selective manner. The researchers also used ultraviolet/visible absorption spectroscopic and radical clock studies to gain insights into the mechanism of these transformations.
Prof Koh said, “This catalyst- and transition metal-free method effectively overcomes previous limitations in scope, scalability and glycosyl donor instability.”
“We expect this general class of glycosyl precursors and their newly discovered reactivity under visible light illumination to find extensive utility in various carbohydrate synthetic applications, enhancing efforts towards the discovery of new sugar-based therapeutics and our understanding of biological processes,” added Prof Koh. The research team plans to work with companies to utilise these findings for the synthesis of sugar-derived compounds. Read the full story here.
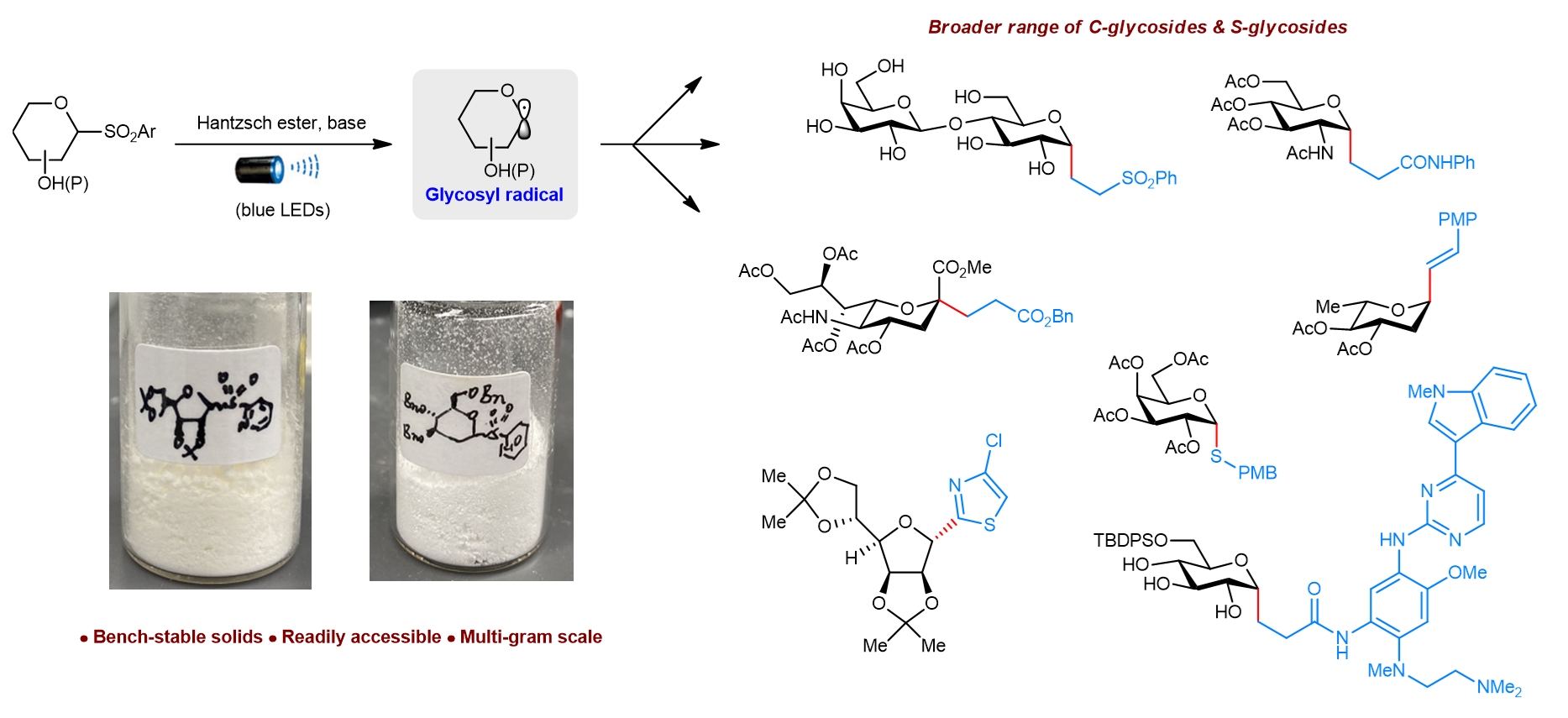
Figure 1: Schematic showing the design of a visible light-mediated system to cross-couple various electrophiles with glycosyl radicals derived from bench-stable heteroaryl sulfones. [Credit: Nature Synthesis]
