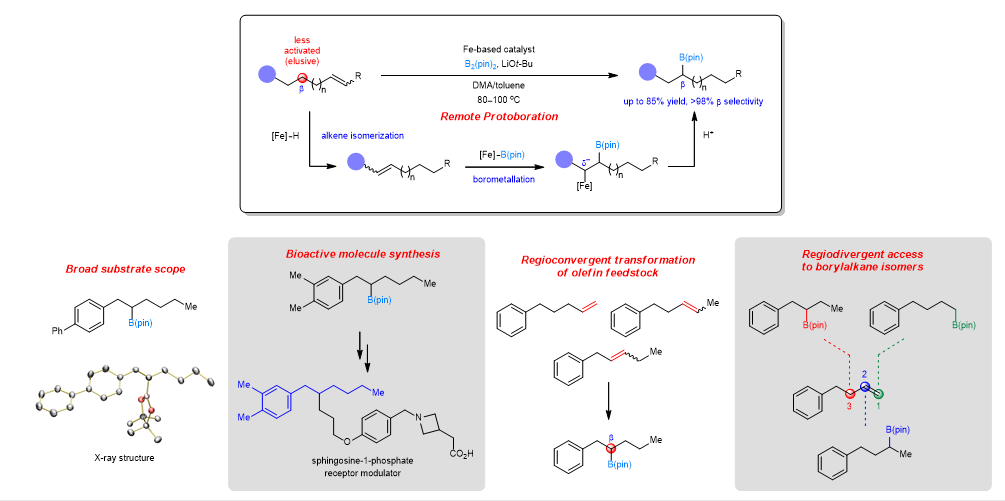
A research team led by Assistant Professor Koh Ming Joo from the Department of Chemistry, NUS has developed a new catalytic transformation known as remote protoboration. Owing to their numerous desirable attributes, boron-containing molecules play a key role in drug discovery and serve as indispensable precursors to diverse chemical products ranging from pharmaceuticals to polymeric materials. In this context, an attractive approach to access aliphatic organoboron compounds involves catalytic hydroboration or protoboration of C-C double bonds, reactions in which hydrogen and boron are both added to an alkene. A modern variation of this process involves remote borylation, whereby boron is inserted distant from the initial reactive site. However, existing methods often lead to borylations at the terminus or at activated positions geminal (α) to functional units. Installing the coveted boryl group at unactivated positions is a formidable challenge. In the new catalytic transformation (remote protoboration) developed by the team, a single iron-based complex promotes alkene isomerization followed by site-selective proto-boryl addition to the C=C bond. Thus, boron can be installed at typically less-activated positions vicinal (β) to common functional groups. By tuning the two processes of alkene transposition and protoboration, the present Fe-catalysed protocol can provide selective access to 1-boryl-, 2-boryl- or 3-borylalkane isomers. The insights gained from this study are expected to advance general efforts towards unlocking selective functionalizations at other unactivated sites along the hydrocarbon chain. This work is published in Nature Catalysis. Read more about their new discovery here.
