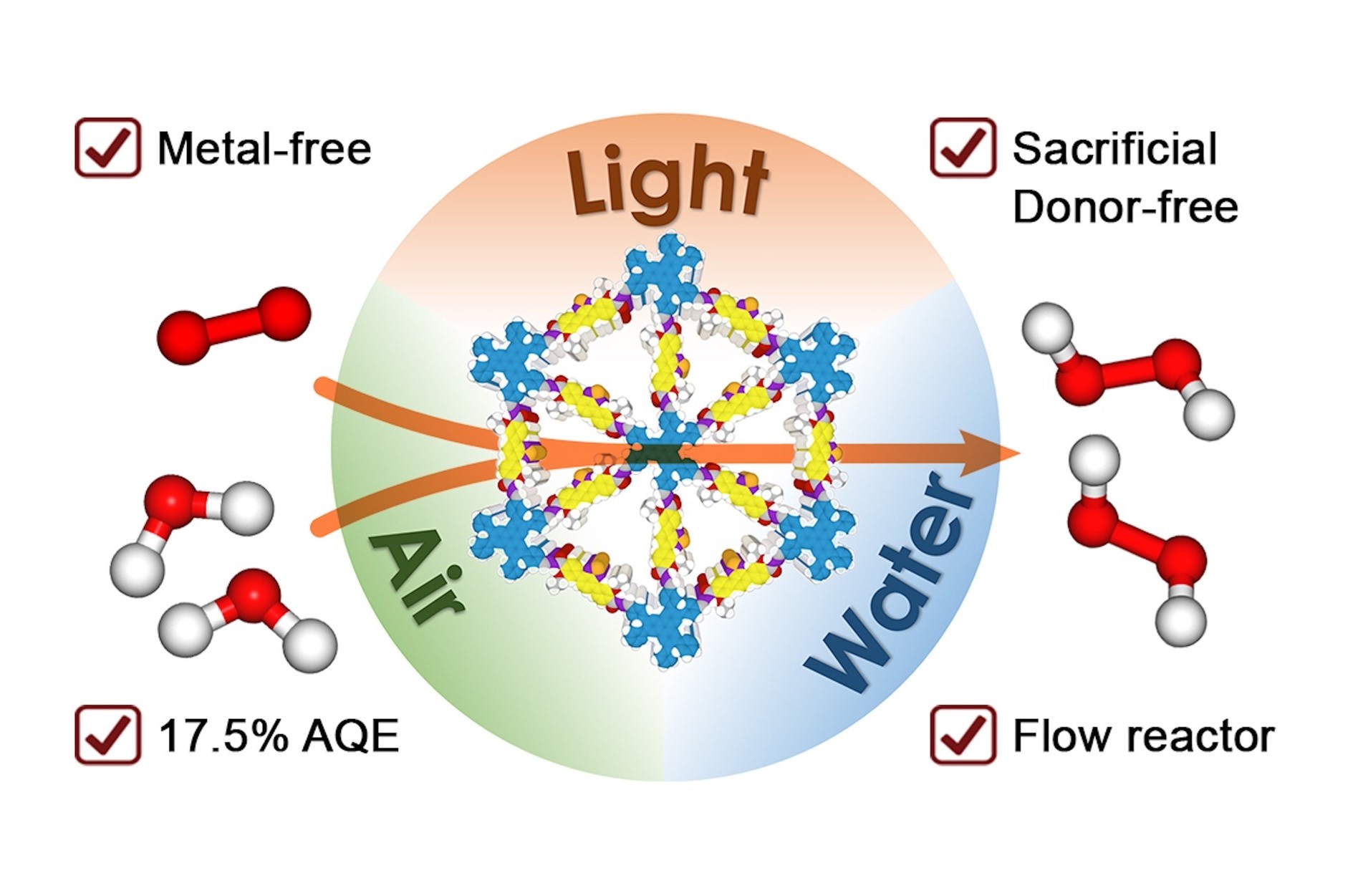
A research team led by Professor Donglin JIANG from the Department of Chemistry, NUS has developed a microporous covalent organic framework with dense donor–acceptor lattices and engineered linkages for the efficient and clean production of hydrogen peroxide (H2O2) through the photosynthesis process with water and air.
Traditional industrial production of H2O2 via the anthraquinone process using hydrogen and oxygen, is highly energy-intensive. This approach employs toxic solvents and expensive noble-metal catalysts, and generates substantial waste from side reactions. In contrast, photocatalytic production of H2O2 from oxygen and water offers an energy-efficient, mild and clean route. Most importantly, it addresses the common drawbacks of existing photocatalytic systems, such as low activity, heavy use of additional alcohol sacrificial donors, and the necessity for pure oxygen gas input.
The research findings were published in the journal Nature Catalysis.
Prof Jiang said, “In this work, we successfully addressed a key and common issue in photocatalysts, electrocatalysts and heterogeneous catalysts, which is the efficient supply of charges and mass to catalytic sites. Our focus on precise structural design at the atomic level to explore both the skeletons and pores of COFs has led to the creation of an artificial photosynthesis system for H2O2 production, achieving unprecedented photocatalytic efficiency.”
Read the full article here.
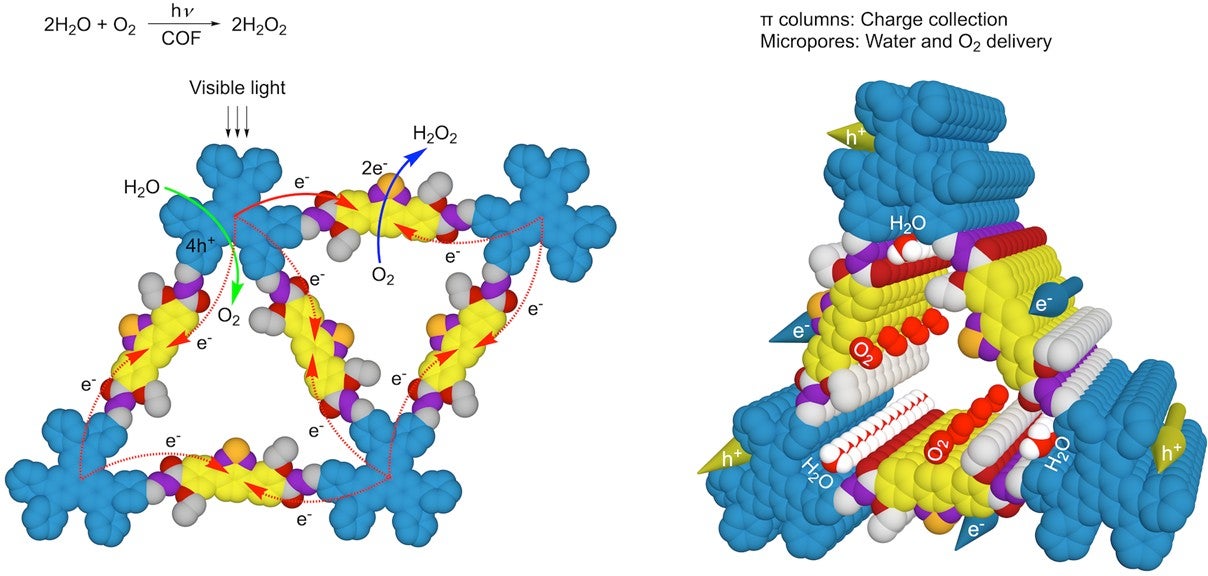
The illustration shows a newly designed hexavalent covalent organic framework (COF) material that mimics photosynthesis.
(Left figure) Light triggers the transfer of an electron from a donor site to an acceptor site within the material (indicated by red arrows). This process transfers four positive charges to the donor site, which are then used to split water molecules into oxygen (indicated by green arrows). At the acceptor site, two electrons combine with oxygen to produce hydrogen peroxide (indicated by blue arrow).
(Right figure) The structure of the material allows for efficient movement of electrons (shown in yellow), positive charges (shown in blue), water, and oxygen throughout the single layer. This material has the potential to convert light energy into chemical energy in a similar way to natural photosynthesis. [Credit: Nature Catalysis]
