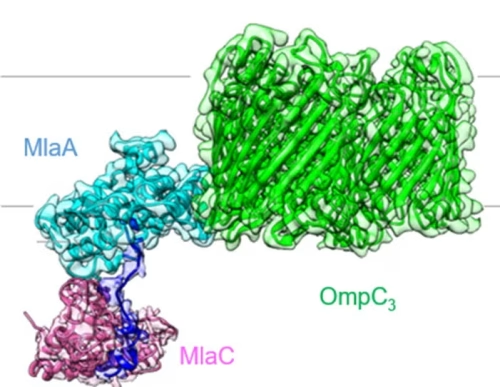
Research teams led by Associate Professor CHNG Shu Sin at the Department of Chemistry, NUS, and the Singapore Centre for Environmental Life Sciences Engineering (SCELSE-NUS), have successfully applied cryo-electron microscopy (cryo-EM) to unveil the molecular structures of critical protein machines that transport lipids and maintain the outer membrane (OM) barrier of Gram-negative bacteria.
Applying single particle cryo-EM in combination with biochemical characterisation, the teams solved the molecular details of the OmpC3-MlaA-MlaC and TolQ5R2A membrane protein complexes isolated from E. coli. They found that the OmpC3-MlaA complex deforms the local membrane, loosening misplaced lipids so they passage through MlaA and slip into the carrier protein MlaC. Using two related structures of the TolQ5R2A protein complex, the teams also gained a better understanding of its role as a tiny, molecular motor to transmit force to help the cell maintain lipid balance in the OM.
These findings were published in the scientific journals Nature Communications and the Journal of the American Chemical Society, respectively.
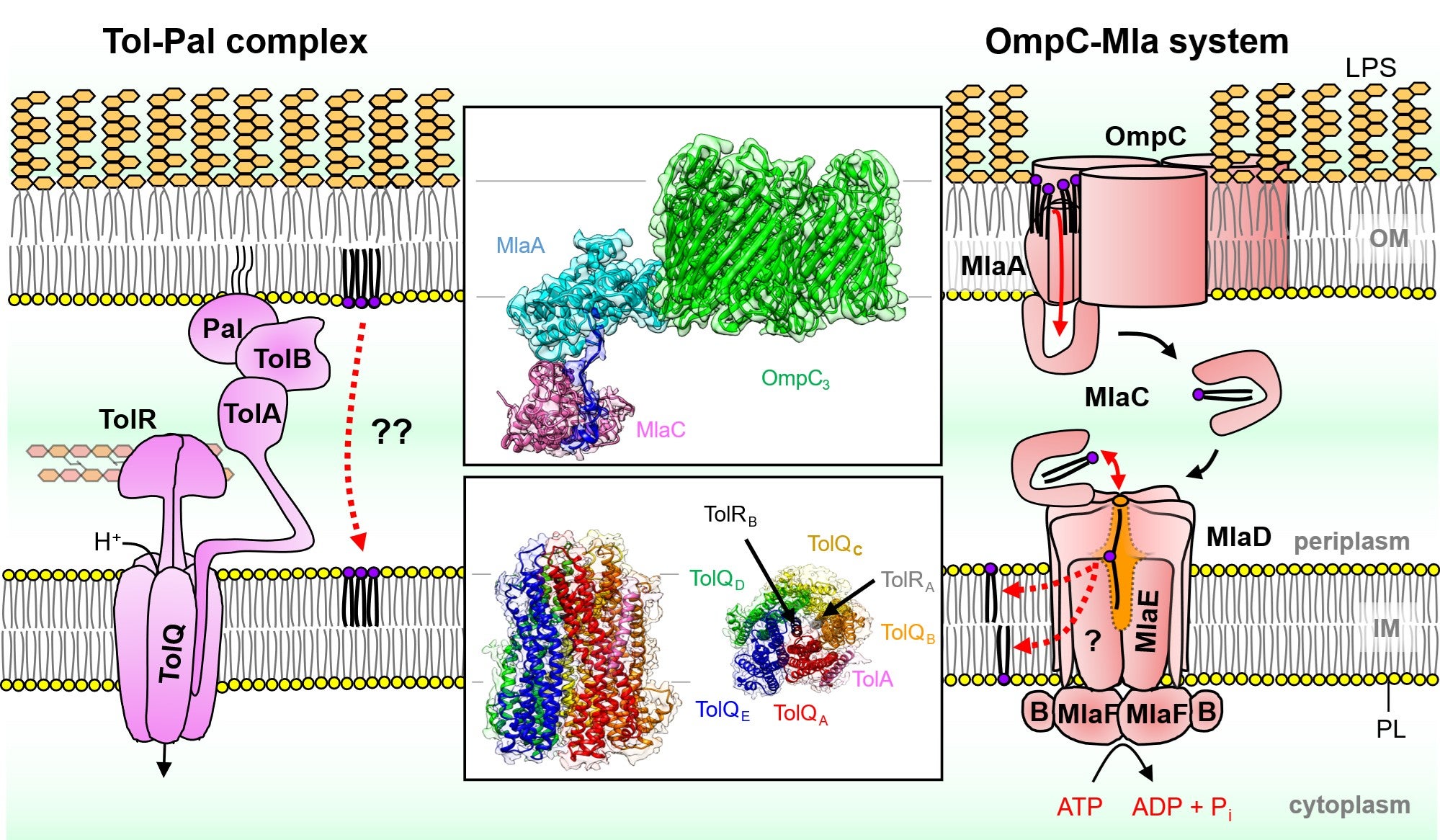
Structural insights into two lipid transport systems in Gram-negative bacteria aid the understanding into their underlying mechanisms. The Tol-Pal complex (left) and the OmpC-Mla system (right) mediates retrograde phospholipid transport to maintain bacterial outer membrane lipid homeostasis and asymmetry, respectively.
“As a biochemistry research group, it is amazing that we can now learn and apply cryo-EM to gain molecular insights into membrane protein machines to elucidate their mechanisms in bacteria, which go a long way towards our fight against drug-resistant infections,” added Prof Chng. Read the full article here.
