
The research team led by Associate Professor LU Jiong, from the Department of Chemistry, developed a scalable hydrothermal approach for obtaining ferromagnetic single-atom spin catalysts which can boost the efficiency of water splitting reactions under the influence of a magnetic field. The major innovation lies in the creation of operando acidic conditions during synthesis that can suppress the aggregation into metal nanoparticles by the dopant. Using this approach, the dopant is able to disperse itself as single atoms evenly on the material to achieve high-density substitution, which is important for the proper formation of the spin catalyst and to provide the ferromagnetic effect. This is a collaboration with Professor Xin LUO from Sun Yat-sen University, China, Dr Shibo XI from Institute of Sustainability for Chemicals, Energy and Environment, Singapore and Professor Cheng-Hao CHUANG from Tamkang University, Taiwan.
This research breakthrough was published in the journal Nature Nanotechnology.
Prof Lu said, “This finding reveals that ferromagnetic SASCs provide a powerful magnetoelectric effect to accelerate water electrolysis. The approach offers unprecedented opportunities for the design of non-precious metal-based ferromagnetic SASCs for both water and saline water electrolyser technologies. We plan to work on large-scale synthesis of ferromagnetic single-atom spin catalysts combined with magnetic fields to develop solutions that can reduce the cost and improve the efficiency of green hydrogen production from the electrolytic splitting of water and seawater.
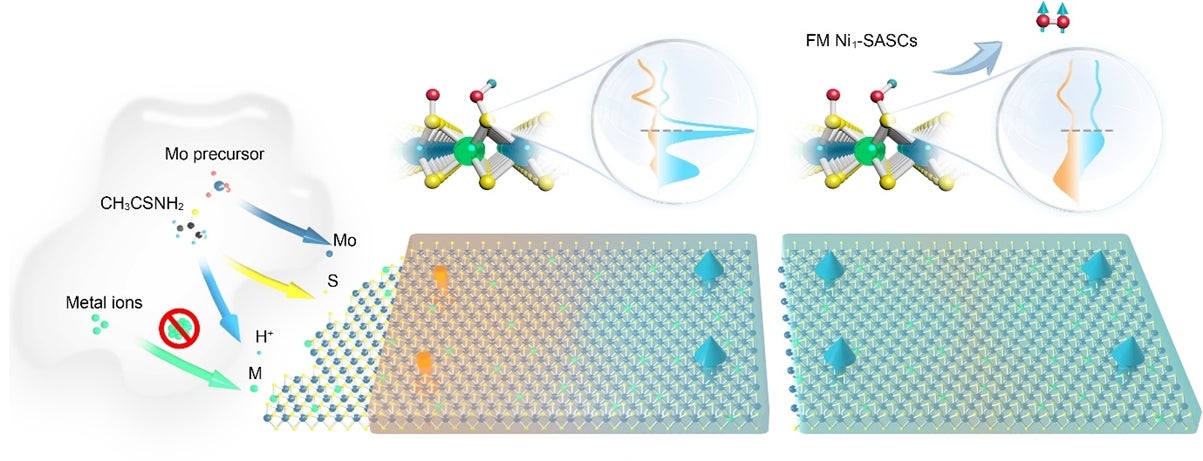
(Left) Illustration of the hydrothermal synthesis of M1/MoS2 single-atom spin catalysts (SASCs) under acidic conditions and (right) the giant magnetic field enhancement of ferromagnetic SASCs used to improve the efficiency of the water splitting reaction. [Credit: Nature Nanotechnology]
