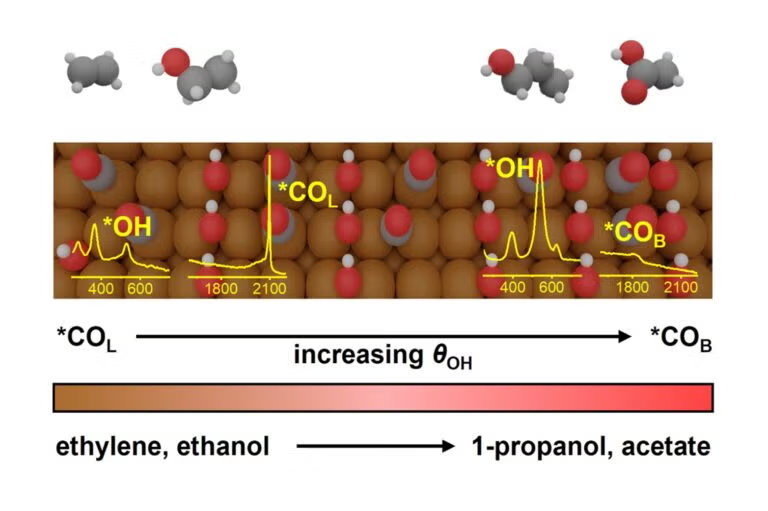
A research team led by Associate Professor YEO Boon Siang, Jason from the Department of Chemistry at NUS has unravelled the reactivity of these differently-adsorbed *CO intermediates in CO2 or CO reduction processes. By combining in-situ Raman spectroscopy, isotope-labelling studies, product analyses, and density functional theory (DFT) simulations, they showed that *COB selectively reacts to form acetate and 1-propanol, while *COL favours ethylene and ethanol formation. Furthermore, they also found that adsorbed *OH domains, which are regions on the copper catalyst surface where hydroxyl groups are adsorbed can displace *COL to *COB, influencing the reaction pathways. This indicates that the selectivity of CO2 or CO reduction could potentially be controlled by tuning the catalyst properties and local environment. The research was conducted in collaboration with Professor Núria López from the Institute of Chemical Research of Catalonia, Spain.
Prof Yeo said, “This work is the fruit of an intense collaboration between experimentalists and theoreticians to discover pathways to selectively produce valuable chemicals, such as ethanol, acetate, and 1-propanol from carbon dioxide reduction.” These insights not only advance the fundamental understanding of the mechanistic pathways of electrochemical CO2 or CO reduction reactions, but also highlight the potential for optimising reaction conditions or catalysts to favour the production of specific multi-carbon products. The findings were published in the Journal of the American Chemical Society. Read the full article here.
| Figure shows the correlation between an increase in OH coverage (θOH), the shift in *CO adsorption configuration from *COL to *COB, and the resulting changes in selectivity from ethylene and ethanol production to acetate and 1-propanol. Unfavoured pathways are indicated by the dotted lines. [Credit: Journal of the American Chemical Society] |  |
