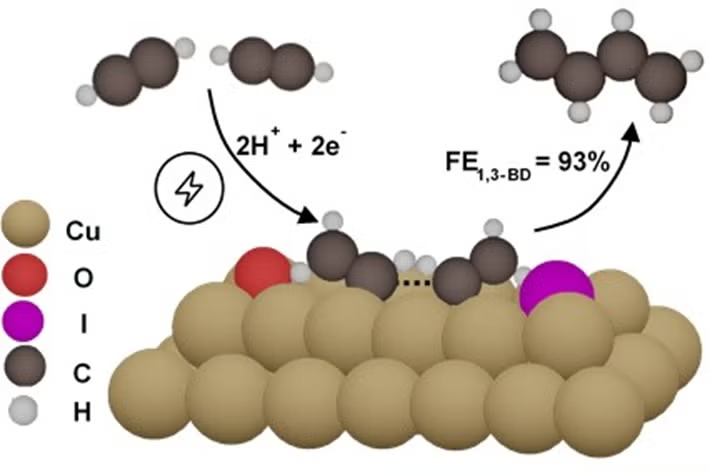
A research team led by Associate Professor YEO Boon Siang, Jason from the Department of Chemistry at NUS has developed a sustainable method to electrosynthesize 1,3-butadiene, a feedstock used for synthetic rubber production, from acetylene. They found that copper catalysts, after a simple modification with iodide anions, are highly efficacious for electro-converting acetylene to 1,3-butadiene at ambient temperature and pressure. 1,3-butadiene could be produced with a Faradaic efficiency of 93% at −0.85 V versus the Standard Hydrogen Electrode (SHE) and a partial current density of −75 mA cm-2 at −1.0 V versus SHE. The partial current density of 1,3-butadiene, an indicator of catalytic activity, was at least 20 times higher than that reported in previous studies. This research was conducted in collaboration with Dr Federico CALLE-VALLEJO from the Basque Foundation for Science and the University of the Basque Country, both in Spain. The team also included Dr Wei Jie TEH from the Department of Chemistry, NUS, Ms Eleonora ROMEO and Professor Francesc ILLAS from the University of Barcelona, Spain, Dr Ben ROWLEY from Shell Global Solutions International B.V., and Dr Shibo XI from the Institute of Sustainability for Chemical, Energy and Environment, Agency for Science, Technology and Research.
Prof Yeo said, “This work is the fruit of an intense collaboration between experimentalists and theoreticians, together with our industrial partner, to discover how important chemicals, such as 1,3-butadiene, could be more sustainably produced.” Building on the research findings from their work, the research team plans to develop catalysts capable of coupling acetylene into longer-chain hydrocarbons, which could potentially be used as aviation fuel.
The findings were published in the journal Nature Catalysis. Read the full article here.
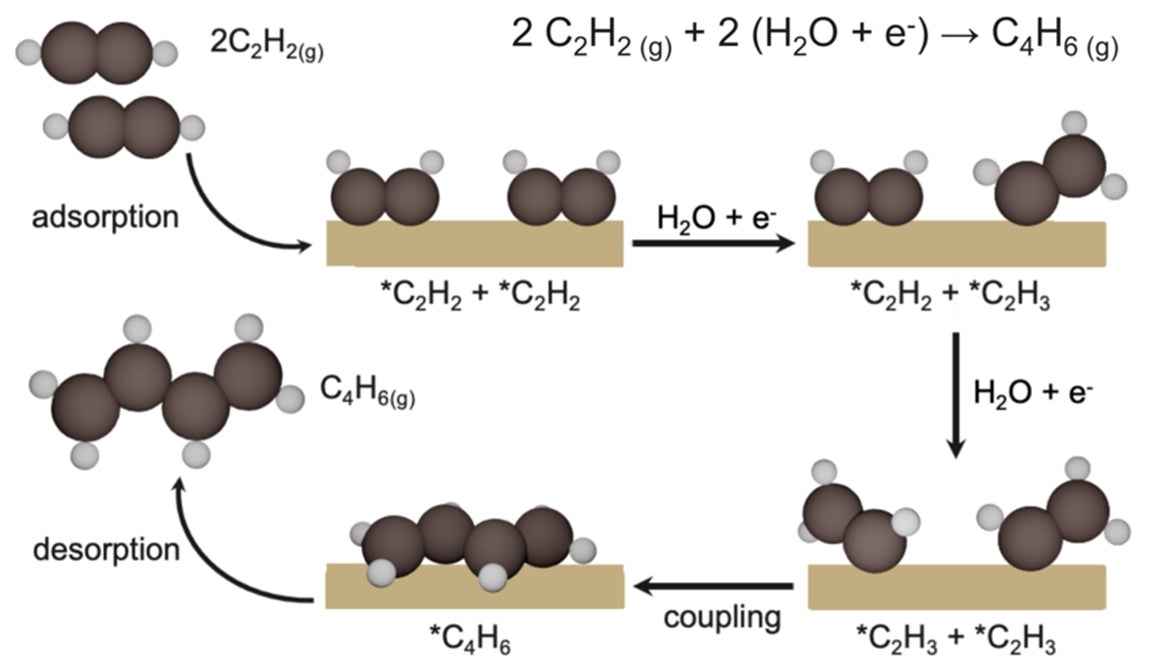
Illustration of the electrocatalytic conversion of acetylene (C2H2) to 1,3-butadiene (C4H6) on copper catalysts. The process involves the adsorption of acetylene molecules onto the catalyst surface, followed by hydrogenation and coupling of *C₂H₂ and *C₂H₃ intermediates, leading to the formation of 1,3-butadiene, which is subsequently desorbed. [Credit: Nature Catalysis]
