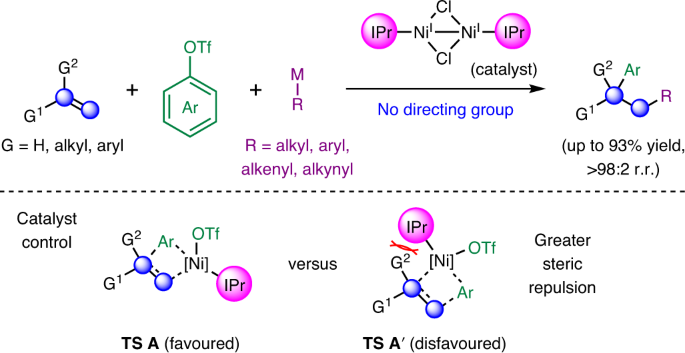
A research team led by Prof KOH Ming Joo, from the Department of Chemistry, National University of Singapore together with researchers at the University of Maryland have conceived a new strategy to drive smoother path to create new C-C bonds using Ni carbene catalyst. They have developed a novel way to make new carbon-carbon bonds without installing directing groups, tiny signposts that tell a functional group where to attach to a molecule. Using a nickel catalyst with a bulky carbene as a ligand, they were able to add two types of carbon functional groups across alkenes, while controlling where each new carbon group ended up.
In the absence of directing auxiliaries, the catalytic addition of carbogenic groups to unactivated alkenes with control of regioselectivity remains an ongoing challenge in organic chemistry. Here they describe a directing-group-free, nickel-catalysed strategy that couples a broad array of unactivated and activated olefins with aryl-substituted triflates and organometallic nucleophiles to afford diarylation adducts in either regioisomeric form, in up to 93% yield and >98% site selectivity. By switching the reagents involved, the present strategy may be extended to other classes of dicarbofunctionalization reactions. Mechanistic and computational investigations offer insights into the origin of the observed regiochemical outcome and the utility of the method is highlighted through the concise syntheses of biologically active molecules. The catalyst control principles reported are expected to advance efforts towards the development of general site-selective alkene functionalizations, removing the requirement for neighbouring activating groups (see the figure above).
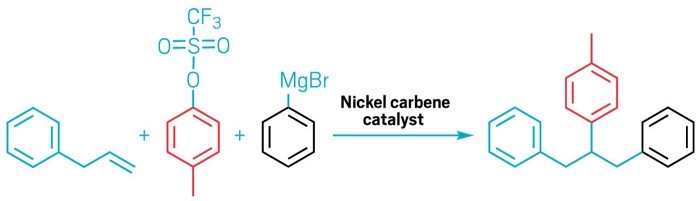
The article was also recently highlighted in C&EN (20 December 2021) – “…This new procedure can potentially shorten syntheses of organic compounds, saving time and money. Koh’s team’s catalyst can aid in adding a variety of functional groups regioselectively across unactivated alkenes. Unactivated means the alkene doesn’t have substituents pulling the bond’s electrons one way or the other, so both carbons in the double bond look similar to incoming functional groups. That’s why chemists have needed to use directing groups, Koh explains. “But then you have to remove them at the end of the day, or transform them to something else,” he says. “So now we can just use any alkene we desire to do the reaction directly.” The group incorporated an N-heterocyclic carbene to the Ni catalyst, which is key in two ways. First, the carbene drives an efficient C-Ni addition across the alkene. “Second, because it is staggeringly large,” Koh says, it forces the Ni catalyst to bind the alkene so that the toluol group adds first (shown below in red), followed by the phenyl group (shown in black). The group made over 50 compounds, by starting with a variety of alkenes, including ones with aromatic and aliphatic groups as well as alkenes with biologically active compounds obtained from a Vitamin E derivative and the lipid drug gemfibrozil. Koh thinks the concept will work for other types of alkene addition reactions and will be important for chemists producing drug-like compounds and building blocks, “because we can generate molecular complexity in a much faster way.”
Read the full story here.
