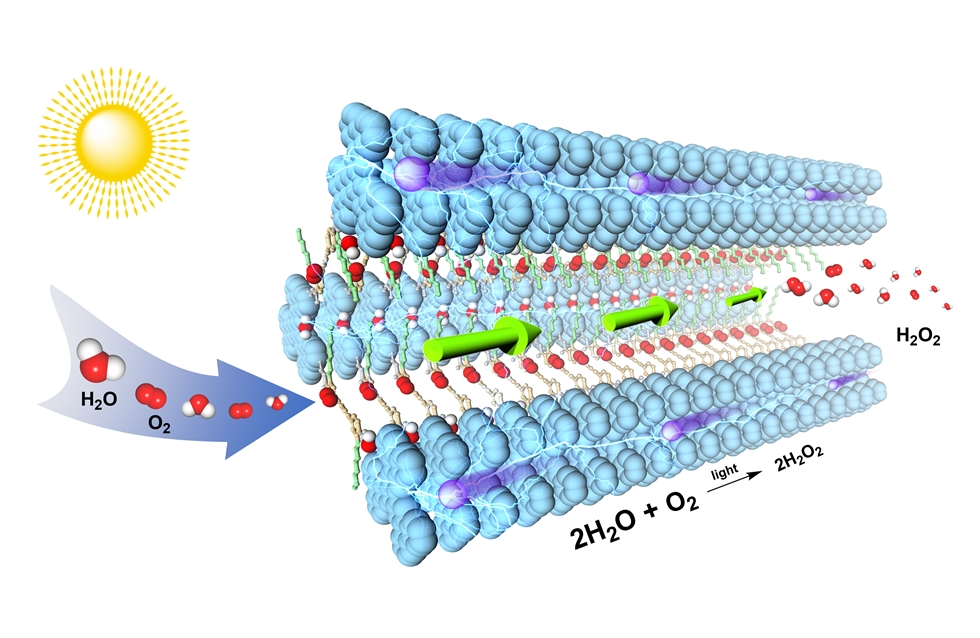
The research team, led by Professor Donglin JIANG from the Department of Chemistry at NUS has developed hexavalent photocatalytic covalent organic frameworks (COFs) which mimic natural photosynthesis via systematic design of the π skeletons and pores for the production of hydrogen peroxide (H2O2), an important industrial chemical. These COFs are porous, crystalline materials built from organic molecules linked together by strong covalent bonds. Their inherent flexibility makes them an ideal platform for constructing photocatalysts. The researchers created a new type of donor-alt-acceptor framework photocatalysts that, upon irradiation, are converted into catalytic scaffolds with dense catalytic sites for oxygen reduction and water oxidation. These photocatalysts possess spatially segregated donor and acceptor columns for holes and electrons separation to prevent charge recombination and enable rapid charge transport. Moreover, the pore walls of the photocatalytic COFs are engineered to be hydrophilic to facilitate water and dissolved oxygen to pass through the 1-dimensional channel to reach the catalytic sites via capillary effect.
Prof Jiang said, “This work embodies nearly two decades of our collective efforts in the field of COFs, culminating in the development of novel photocatalysts that effectively address two fundamental yet formidable challenges: the simultaneous and efficient delivery of charges and mass to catalytic sites. The breakthroughs presented herein signify a compelling and paradigm-shifting advancement in the realm of artificial photosynthesis.” Read the full article here.
The research findings were published in the journal Nature Synthesis.
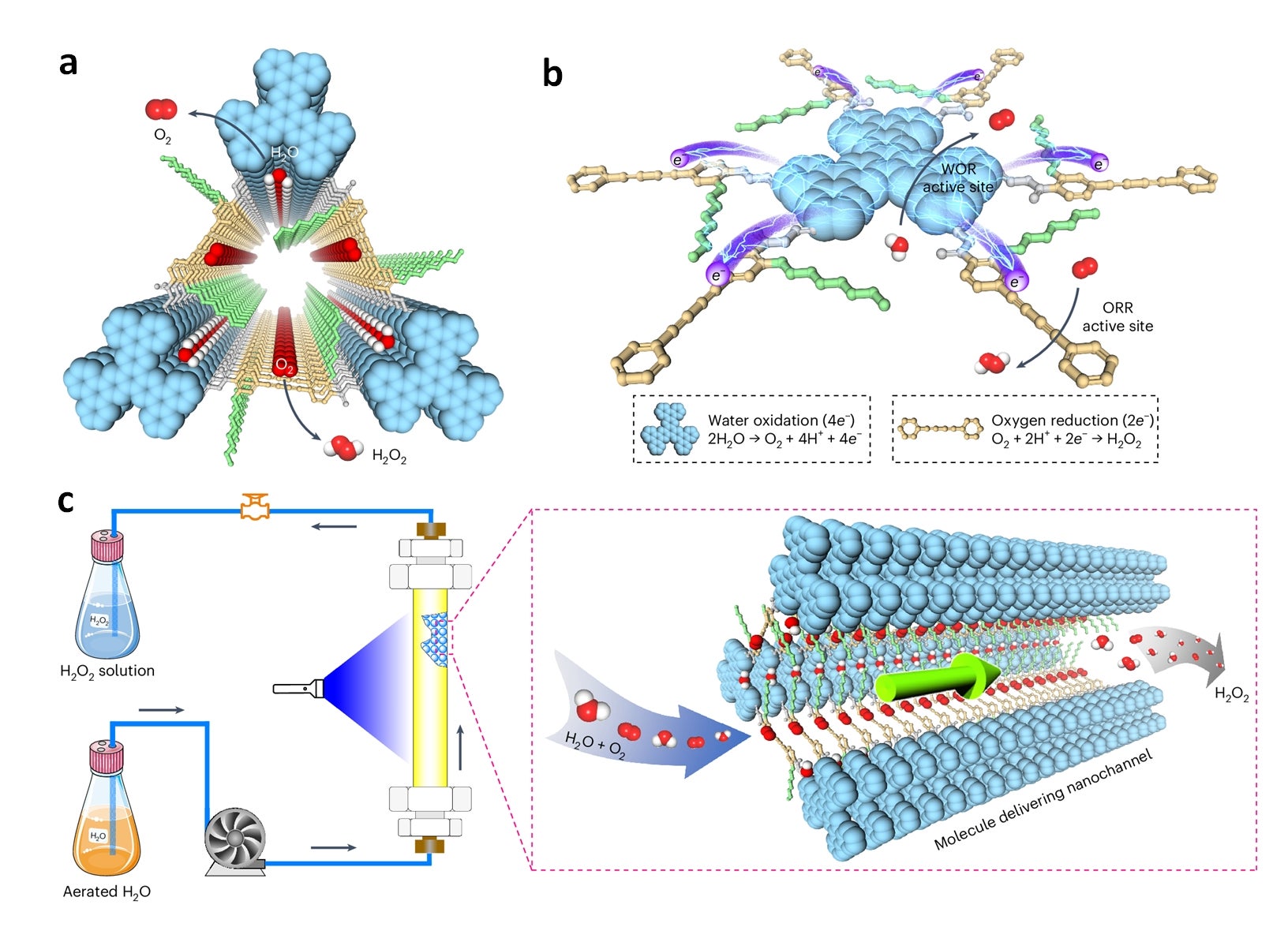
Figure shows the systematic design of the photocatalytic covalent organic frameworks (COFs) for manufacturing hydrogen peroxide (H2O2) using flow reactors. (a) The COF with segregated donor and acceptor zones along with specially designed water channels. (b) Under light, charges separate, with oxygen reduction occurring at one region, and water oxidation at another region. (c) Continuous production of H2O2 occurs in a flow reactor, where water and oxygen transform into H2O2 through the tiny channels of the photocatalyst. [Credit: Nature Synthesis]
