A research team led by Professor LIU Xiaogang from the Department of Chemistry at NUS, leveraged on rare-earth X–ray absorption and ligand-mediated triplet exciton harvesting to overcome these challenges and significantly improved the performance of molecular scintillators. The effective trapping of the energy dissipated during secondary X-ray relaxation via organic ligands led to a remarkable 1,300-fold increase in radioluminescence compared to lanthanide salts. The study unveiled the role of triplet exciton recycling in determining scintillation efficiency, demonstrating that high photoluminescence quantum yield may not necessarily result in high scintillation efficiency. This research was conducted in collaboration with Professor Yiming WU from Xiamen University, China and Professor Xian QIN from Fujian Normal University, China.
Prof Liu said, “The efficiency of triplet exciton recycling holds the key to better scintillation performance. These discoveries lend profound insights into X-ray-induced exciton migration dynamics and radioluminescence behavior, shaping the future of organic scintillators and their harnessing of high-energy X-ray quanta.” “The high stability of radioluminescence, large Stokes shift and full spectral tunability make organolanthanide molecules a promising platform for scintillation applications,” added Prof Liu. The findings were published in the journal Nature Photonics. Read the full article here.
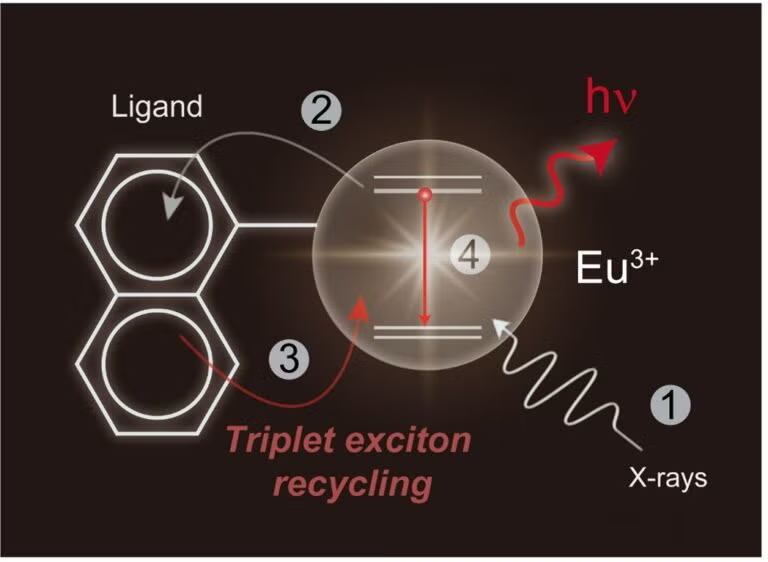
Figure above describes the process of triplet exciton-mediated energy transfer from molecular antenna to lanthanide centres (Eu³⁺). X-ray irradiation (1) triggers a cascade of secondary X-ray excitations, which are subsequently captured by organic ligands (2) after thermalisation and subsequently transferred back to the lanthanide centres via resonance energy transfer (4), resulting in enhanced radioluminescence (hv). [Credit: Nature Photonics]
