
A research team led by Associate Professor KOH Ming Joo, from the Department of Chemistry, NUS has solved a longstanding challenge in the synthesis of congested C(sp3)-rich molecules by developing a new iron-catalysed reaction that generates two alkyl-alkyl bonds in crowded environments.
His team conceived a new strategy that harnesses an earth-abundant (terpyridine) iron catalyst to combine alkenes with sp3-hybridised organohalides and organozinc reagents. This approach allows them to add different-sized alkyl groups to the alkene, resulting in a library of drug-like molecules with congested cores containing either carbon- or heteroatom-substituted stereocenters. The method is useful for creating valuable but challenging C(sp3)-rich molecules. This research is a collaboration with Dr Xinglong ZHANG from the Institute of High Performance Computing, Agency for Science, Technology and Research (A∗STAR) and Professor Patrick HOLLAND from Yale University.
The findings were published in Nature Catalysis.
Prof Koh said, “Our studies suggest that this iron-catalysed dialkylation reaction operates through a unique mechanism, which potentially opens the door to a wider range of transformations and new chemical space. This helps to diversify and broaden the chemical structures of performance molecules.”
“We believe this method will accelerate the synthesis of many natural products and pharmaceuticals in a sustainable manner, especially those that contain densely functionalised alkyl-alkyl linkages,” added Prof Koh.
The research team is leveraging the newly discovered iron-catalysed system to transform other classes of organic starting materials into useful compounds for various applications, including drug development. Read the full article here.
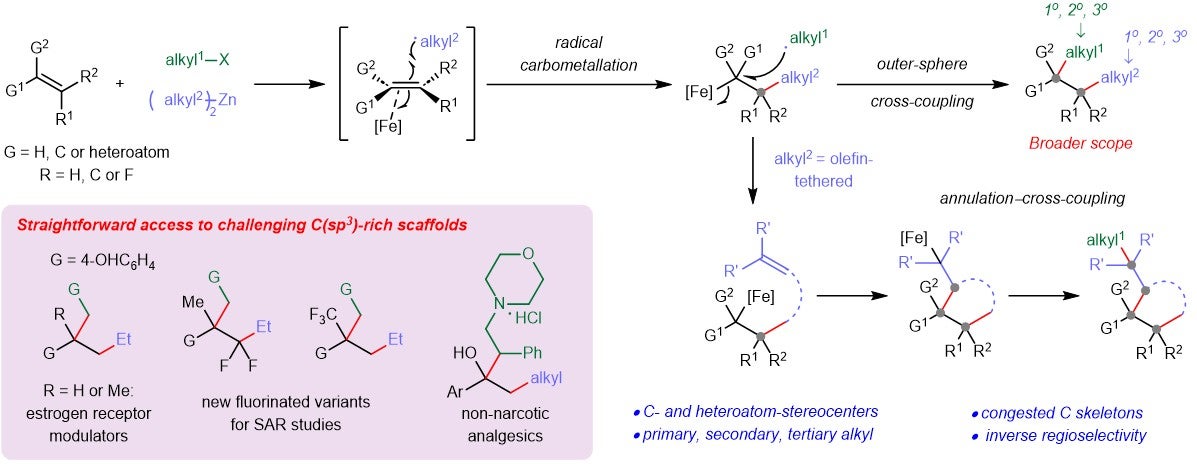
Figure: Schematic showing the design of a new iron-catalysed dialkylation reaction that transforms abundant alkenes to prized C(sp3)-rich molecules. [Credit: Nature Catalysis]
