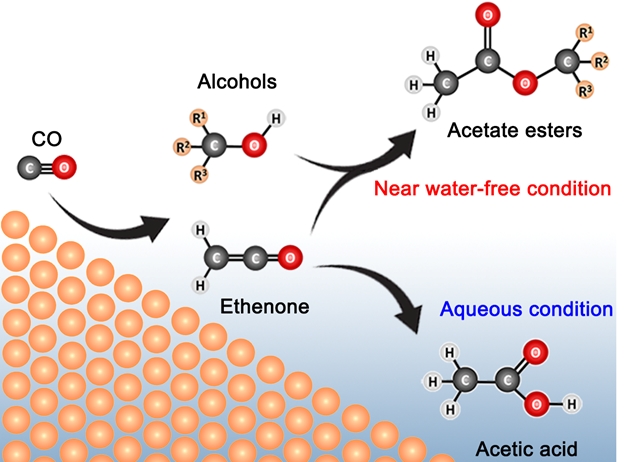
A research team led by Associate Professor YEO Boon Siang, Jason from the Department of Chemistry, NUS in collaboration with Dr Sumit VERMA and Dr Ramesha GANGANAHALLI from the international energy company Shell has discovered how C3 – C6 acetate esters can be electro-synthesised from carbon monoxide and water using copper catalysts in a membrane electrode assembly. Ethyl acetate and propyl acetate can be produced with a total Faradaic efficiency (FE) of about 22% and with a current density of up to 55 mA/cm2, alongside minor quantities of methyl acetate and butyl acetate. The esters were produced via the addition reaction of ethenone (H2C=C=O) and alcohols produced during CO reduction (see Figure). The near water-free reaction condition and the high local pH level play key roles in the formation of the esters.
NUS and Shell had agreed earlier last year to jointly develop processes to create green fuels and chemicals for the energy industry (see link).
Prof Yeo said, “This work is a result of the collaboration between NUS and Shell to sustainably create chemicals. Our team has succeeded in developing an electrochemically-driven process to make esters using renewable feedstocks such as carbon monoxide and water.”
The above figure shows the processes involved in the production of C3 – C6 acetate esters from the electrocatalytic reduction of carbon monoxide in a membrane electrode assembly cell. [Credit: ANGEWANDTE CHEMIE INTERNATIONAL EDITION]. Read the full story here.
