The World is facing many challenges today, one of the most dangerous is the climate change. The other critical challenges include the sustainability of our ever-increasing energy demand, the sustainable quality of our air, water and food, and the need to conserve Earth’s resources while developing products and technologies that will continue to advance the society.
Chemical manufacturing is one of the central pillars of global economy and has played a key role in the advancement of society. Indeed, we believe that chemistry is the key to resolve many of these problems – to lower carbon emission, to develop renewable resources and energy-efficient technologies. For example, chemistry can work towards the upcycling of plastics instead of a mere recycling of the materials. Also, the use of catalysis to accelerate chemical processes can reduce much of the energy, time and resources needed as well as the waste by-products generated. Chemists all over the world have started earnest search for sustainable and cost-effective alternatives and solutions for problems facing us today and in the future.
A good foundation and understanding in Chemistry empower us to create better sustainable solutions to resolve environmental issues, pharmaceutical breakthroughs, materials upcycling, energy efficiency, etc. In this effort, NUS Department of Chemistry has put together “The solutions are in Chemistry” lecture series, as we hope to share with our younger generations the beauty and potentials of chemistry.
For teachers who are interested to organise one of these lectures for students, please write to chemistry-outreach@nus.edu.sg and give several date/time options.
Energy is essential for modern society to function. Human civilization has undergone a series of energy transitions, evolving from reliance on wood as a primary fuel source to coal, and subsequently shifting towards petroleum and natural gas as dominant energy resources.
Natural gas (methane being the major constituent) is currently the cleanest fossil fuel in terms of CO2 emissions and its use for power generation in electricity grids worldwide has been steadily increasing. In Singapore, the bulk of our electricity presently is generated by combusting natural gas in Combined Cycle Gas Turbines (CCGTs).
Hydrogen is often touted as the fuel of the future as its only emission from combustion is water. In Singapore, we are striving to develop a hydrogen economy by 2050 [1] to fulfil our net zero commitments. There are many diverse ways to obtain hydrogen with different technology levels and carbon footprint such as steam methane reforming, electrolysis, photocatalysis, thermal water splitting and gasification.

In this talk, I shall elaborate on the intimate relationship between hydrogen and methane, and highlight that mastery of their synthesis, interconversion and storage of these two gases will be key to our sustainable energy future.
[1] https://www.channelnewsasia.com/commentary/net-zero-target-hydrogen-strategy-research-trial-clean-energy-climate-change-3026426
.
 | Dr Foo Maw Lin was born in Singapore. He did his BSc (Hons) and MSc in NUS Chemistry. This was followed by a PhD in Chemistry and Materials at Princeton University, USA. He then performed research in US, Canada and Japan. Dr Foo joined NUS Chemistry in 2015 as a member of the teaching staff and is currently a senior lecturer. He is currently teaching courses in renewable energy and electric vehicles. His pedagogical interests are in interdisciplinary education and game-based learning. |
Catalysis is an indispensable process that drives chemical manufacturing and enable access to various categories of products, ranging from fine chemicals, active pharmaceutical ingredients, to polymeric materials. Despite advances made in this field, many reactions still rely on catalysts derived from rare and expensive precious metals such as palladium. Since most precious metal-derived catalysts can only mediate a narrow range of chemical reactions, longer synthetic sequences often have to be employed to convert a starting material to the desired target product. Each step in a chemical synthesis process consumes energy, resources and time, and generates spent waste solvents and other byproducts. Based on the NEA’s statistics, millions of liters of spent solvents are collected annually in Singapore and subsequently incinerated, which leads to more carbon dioxide and other toxic emissions that contribute to a series of negative repercussions on our environment.
Base metals such as nickel are earth-abundant, inexpensive and less toxic. For example, nickel is 5,000 times more abundant and at least 2,000 times cheaper than palladium. In addition, base metals exhibit distinct reactivity and selectivity profiles that enable them to mediate new chemical transformations that provide new opportunities to assemble molecules in a cheaper and faster manner. Therefore, base metal catalysis has tremendous potential to drive sustainable chemical production beyond the limits of traditional precious metal catalysts. In this context, Prof Koh Ming Joo’s research group has built a program on sustainable catalysis where new multitasking and energy-efficient base metal catalysts were developed to mediate unprecedented chemical reactions, significantly enhancing the efficiency and shortening the required steps to make a target product. By combining with reactivity-tunable radical chemistry strategies, they employ these catalysts in innovative approaches to transform cheap and abundant feedstock chemicals to value-added chemicals with a lower carbon footprint. In this lecture, Prof Koh will share these exciting chemistry and research outcome of his group.
 | Associate Professor Koh Ming Joo was born and raised in Singapore. He received his B.Sc. (First Class Honours) degree in Chemistry & Biological Chemistry from NTU in 2012, before heading to Boston College for his Ph.D. and post-doctoral studies under the supervision of Professor Amir Hoveyda from 20122018. In June 2018, Koh joined NUS Chemistry as a President’s Assistant Professor, and was promoted to Associate Professor in July 2023. His current research focuses on developing sustainable and practical catalytic solutions that address critical challenges in chemical synthesis through base metal catalysis and radical chemistry. His work has been published in reputable scientific journals such as Nature Catalysis and Nature Chemistry. A/P Koh is a recipient of the NUS Inauguration Grant (2019), Asian Core Program Lectureship Awards for Japan, Korea, China and Thailand (2019, 2022), Innovators Under 35 (TR35) Asia Pacific Award (2021), TCI-SNIC Industry Award in Synthetic Chemistry (2021), Thieme Chemistry Journals Award (2022), C&EN’s Talented 12 Award (2022), Young Scientist Award, SNAS (2022), Young Researcher Award, NUS (2023) and also the 2023 Novartis Early Career Award in Chemistry. |
The chemical industry is one of the biggest industries in the world, producing a huge range of products, including pesticides for agriculture, lubricants for machinery, ingredients in cleaning agents, cosmetics, pharmaceutical and plastics. However the chemical sector is not always the most sustainable of endeavours. Resource consumption, waste generation and high energy demands have a widespread impact on the environment, including human health, wildlife and natural resources. So
Green/sustainable chemistry is a scientific concept that aims to improve the efficiency with which natural resources are used to meet human needs for chemical products and services. It encompasses the design, synthesis and use of efficient, effective and more environmentally benign chemical products and processes. However, achieving truly green synthesis is incredibly challenging and through the years various approaches that offer a greener means to chemical synthesis via novel synthetic strategies, apparati to simplify operations and greener solvents that are inherently environmentally and ecologically benign have been developed. In this lecture, Prof Lam would be sharing the works from her laboratory in this area of chemistry.
 | Associate Professor Lam Yulin was born and raised in Singapore. She received her undergraduate and graduate studies in NUS, before heading to The Scripps Research Institute for post-doctoral studies under the supervision of Professor Kim D Janda. Upon returning to Singapore, she joined the Institute of Molecular and Cell Biology as a Research Fellow working on DNA Repair. Lam started her own independent research career in NUS 1997. Her current research focuses on (i) synthesis and biological evaluations of novel organic compounds and natural product derivatives as potential therapeutic agents, and (ii) development of recyclable reagents and catalysts for greener organic transformations. |
After three decades of research, organic light-emitting diodes (OLEDs) and semiconductor colloidal quantum dots (QDs) have now emerged as dominant technologies in the colour-display industry. In particular, the societal contributions and impact of QDs have been recognised in the 2023 Nobel Prize in Chemistry.
These materials offer superior display contrast, good electrical efficiency, and thin device profile, which are valuable for smart-phones, televisions and other electronic display applications. Perhaps less known to many, the development of such materials involves much design and careful structural studies in chemistry. In this lecture, Prof Tan Zhi Kuang will highlight the fascinating chemistry behind these exciting and colourful nanomaterials. He will also discuss the potential applications in many emerging technologies, such as in clean energy generation and in autonomous vehicles sensing.
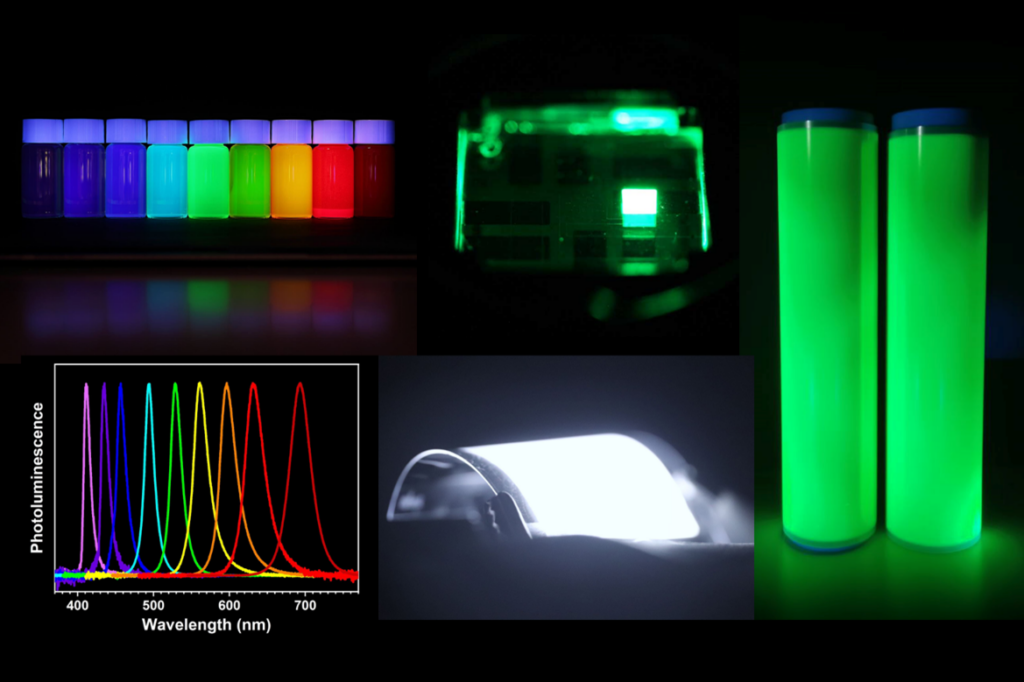
 | Associate Professor Tan Zhi Kuang is born and raised in Singapore. He studied Chemistry and Technology Entrepreneurship at NUS, and received his B.Sc. degree with First-class Honours in 2010. He earned his Ph.D. in Physics at the University of Cambridge through the support of the prestigious Singapore National Research Foundation (NRF) scholarship. During his Ph.D., Prof Tan investigated the physics of optoelectronic devices, with a focus on the heterojunction energetics of organic light-emitting diodes (OLED) and solar cells. In 2014, he invented the first perovskite-based light-emitting diode, which possesses remarkable colour performance and efficiencies, and has since attracted significant academic and commercial interest. His research has been published in reputable scientific journals, including Nature Nanotechnology, Nature Photonics and Advanced Materials, and has received an aggregate of 8,000 academic citations. His research inventions have also led to multiple licensed patents and two deep-tech start-up companies in the advanced display technologies domain. In 2017, he won the NUS Early Career Research Award. Prof Tan currently leads a research group in NUS Chemistry that aims to investigate the rich photo-physics of perovskite semiconductors and quantum dots, and seeks to develop advanced photonic devices for colour displays and wearable electronics applications. |
Plastics is one of the most important and commonly used material in the modern society. The global production of plastic doubles in just 20 years from 200 Metric tonnes (Mt) in 2000 to 400 Mt in 2020. The demand is predicted to continue rising exponentially in the near future.
No doubt plastics bring convenience to our daily life and find countless industrial applications, but plastics pollution is becoming a critical environmental issue worldwide. While the recycling rates of ferrous metal (99%), wood (71%) and paper (37%) are either high or improving, the recycling rate of plastics remains as low as …… 6% ONLY [1] … but WHY?
The traditional recycling of plastics typically produces the same plastic products, e.g. PET (polyethylene terephthalate) plastic bottle is recycled to its constituent monomer(s) and re-polymerized to PET to make a new plastic bottle. This approach is no doubt of sustainable value but generally lacks economic value. This is one major reason contributing to the super low recycling rate of plastics globally.
Upcycling of plastics, on the other hand, can produce products that is more valuable than the original plastics, hence the upcycling process becomes more sustainable both environmentally and economically. Hence, the solution still lies in chemistry!
How do chemists achieve plastic upcycling using their “magical” reactions? Come and join Prof Xi Yumeng to hear the upcycling stories and his research at NUS Chemistry that contributes to the sustainability of not only Singapore but also our Mother Earth.
[1] Key Highlights of the 2022 Waste and Recycling Statistics, National Environment Agency, Singapore.
 | Assistant Professor Xi Yumeng received his B.Sc. degree in Chemistry from Peking University in 2011. He proceeded to pursue his Ph.D. degree under the supervision of Professor John F. Hartwig at the University of California, Berkeley and graduated in 2019. Dr Xi then moved on to carry out his postdoctoral research with Prof Craig J. Hawker at the University of California, Santa Barbara. His research interests are in the field of synthetic organic chemistry, organometallic chemistry, catalysis and polymer science. Dr Xi joined NUS Chemistry as a NUS Presidential Young Professor (PYP) candidate in January 2023. |
The atmospheric CO2 level had been monitored for the past 65 years by NOAA (National Oceanic and Atmospheric Administration) and it is the 11th consecutive year that the level has increased by more than 2 ppm. In 2022, the level rose by 2.13 ppm to 417.06 ppm.
What does CO2 do?
Excess CO2 blankets the earth and traps the heat from Sun, which leads to increased average temperature of earth. The warmer temperatures over time are changing the weather pattern leading to climate change. The ground effects that we experience are severe storms, increased drought, ocean acidification, rising ocean, loss of species and many health risks.
How do we control CO2 emission?
We should stop using plastic, incorporate walking and biking to reduce the usage of fossil fuels, turn off lights/Aircon as much as possible, use food that is grown locally, reduce food wastage, use more renewable energy and green technologies …. these are the control measures, but not so easy to practice. Is there any other action for sustainability?
Yes, there is….
The solutions are in chemistry – what if we can convert the atmospheric CO2 to useful products?
Come and learn about the research work of NUS Chemistry Prof Yeo Boon Siang, Jason. He is working towards the major scientific objective for the 21st century that is to develop a sustainable and environmentally friendly energy economy. His research group develops efficient and robust materials to catalyze energy conversion reactions. The systems of interest include reduction of CO2 to multi-carbon chemicals and fuels such as n-propanol and water splitting. He applies state-of-art analytical for such as operando spectroscopy to understand the mechanistic and structural aspects of these processes. The Knowledge is then be exploited to design and synthesize systems with improved functionality.
 | Associate Professor Yeo Boon Siang, Jason studied chemistry at NUS, where he received his B.Sc. (Hons) and M.Sc. degrees. He obtained his Ph.D. from the ETH Zurich, and did postdoctoral research at the Lawrence Berkeley National Laboratory. Associate Professor Yeo joined NUS Chemistry in 2012. His work focuses on developing efficient and robust materials to catalyse energy conversion reactions to achieve sustainable and environmentally friendly energy economy. Systems of interest include reduction of carbon dioxide to multi-carbon chemicals and fuels such as n-propanol, and water splitting. Associate Professor Yeo has been awarded multiple faculty- and university-level teaching excellence awards. He is currently a Dean’s Chair Professor and the Deputy Head of Education at the Department. |
Over the past few decades, life sciences have witnessed an influx of numerous physico-chemical tools as well as the constant arrival of researchers from the physical sciences. It is increasingly clear that biology has become a quantitative science. As such, understanding the physico-chemical methodologies and principles that underpin life processes becomes more essential than ever, in order for us to navigate this “quantitative turn” in biology.
Biophysical chemistry is an exciting interdisciplinary field that applies fundamental concepts and techniques from physical chemistry to quantitatively probe biomolecular and cellular systems. In particular, fluorescence spectroscopy and imaging provide one of the the most direct, powerful and versatile ways to reveal the inner workings of living systems in both space and time, with high sensitivity, specificity and resolution.
In this talk, I will introduce how recent advancements in fluorescence spectroscopy and imaging, as epitomized by the “GFP revolution”, single-molecule tracking and super-resolution microscopy, have empowered us to illuminate diverse biological processes that take place inside a living cell, beyond the diffraction limit of light, and one molecule at a time.
 | Ziqing Winston Zhao is Assistant Professor of Chemistry at the National University of Singapore. He is also a Principal Investigator at the Centre for BioImaging Sciences and the Mechanobiology Institute at NUS. He received his double B.S. degrees in Chemistry and Biology from Caltech, his Ph.D. from Harvard University with Sunney Xie, and his postdoctoral trainings at the Agency for Science, Technology and Research (A*STAR), before joining the NUS faculty under a NUS Presidential Young Professorship. His research focuses on developing and applying advanced imaging-based approaches to quantitatively probe the biophysics of chromatin organization and dynamics at the single-cell level. |
Catalysis is a great tool in achieving sustainable production of various chemical products, as it improves reaction selectivity, reduces waste and allows the reactions to proceed at milder conditions (i.e. lower temperature and pressure, and less consumption of materials).
As chemistry students, you may have learnt / will learn some of these most famous catalysts: • Fe-based catalyst used for ammonia synthesis in Haber process – Nobel Prize 1918 • Ziegler-Natta catalyst used for synthesis of some most important addition polymers such as polyethene (PE) and polypropene (PP) – Nobel Prize 1963 • Chiral catalysts used in asymmetric synthesis which beautifully controls the stereochemistry outcome of multiple organic reactions – Nobel Prize 2001 & 2021
Enzymes are naturally-occurring biocatalysts that are famous in their ultra-high selectivity (specificity) and reactivity under mild conditions (room temperature/pressure and benign solvent – water). At the same time, DNA has been traditionally considered as ultra-stable molecule that is responsible to store the genetic information, but nothing more.
Have you ever imagined that DNA can be derivatized and become a biocatalyst too? The carefully designed DNA-catalyst can achieve both the high reactivity and a wide reaction scope that catalyzes not only one reaction but one entire class of reactions.
Come and join Prof Zhu Ru-Yi’s talk on DNA catalysis, and learn how DNA-catalyst is designed, synthesized and utilized to improve the sustainability of multiple chemical processes!
 |
Assistant Professor Zhu Ru-Yi received his B.Sc. degree in Chemistry from Peking University in 2013. He then went on to pursue his Ph.D. from The Scripps Research Institute in 2018, under the supervision of Professor Jin-Quan Yu. Subsequently, Dr Zhu had his postdoctoral training at Stanford University, working with Professor Eric Kool on chemical biology of DNA, RNA, and associated proteins for cancer therapeutics. Dr Zhu joined NUS Chemistry in September 2021 under the NUS Presidential Young Professorship. His research integrates state-of-the-art synthetic organic chemistry with molecular biology to validate therapeutic targets, identify potential small-molecule anti-cancer drugs and develop molecular tools for cancer early diagnosis. Specifically, his on-going projects include: (i) next-generation RNA therapeutics, (ii) reprogram RNA with small-molecule and (iii) enzyme-like DNA catalyst design. |
