Postdoctoral, University of California Berkeley/ Scripps Research Institute (USA); PhD, Purdue University (USA); B.Sc., Ohio State University (USA).
Contact Information:
Office: S9-12-01F
Tel: (65)-6516-2669
Fax: (65)-6779-1691
Email: chmyaosq@nus.edu.sg
Chemical biology and medicinal chemistry. We are interested in developing powerful strategies in Chemical Biology and Chemical Proteomics that enable organism-wide, high-throughput studies of enzymes, e.g. the so-called “catalomics”.
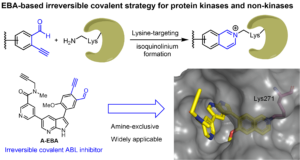
Ref. : Chen, P.; Tang, G.; Zhu, C.; Sun, J.; Wang, X.; Xiang, M.; Huang, H.; Wang, W.; Li, L.; Zhang, Z.-M.; Gao, L.; Yao, S. Q., 2-Ethynylbenzaldehyde (EBA)-based, lysine-targeting irreversible covalent inhibitors for protein kinases and non-kinases. J. Am. Chem. Soc. 2023, 145, 3844-3849.
Lysine-targeting irreversible covalent inhibitors have attracted growing interests in recent years, especially in the fields of ki-nase research. Despite encouraging progress, few chemistries are available to develop inhibitors that are exclusively lysine-targeting, selective, and cell-active. We report herein a 2-ethynylbenzaldehyde (EBA)-based, lysine-targeting strategy to generate potent and selective small-molecule inhibitors of ABL kinase by selectively targeting the conserved catalytic lysine in the enzyme. We showed the resulting compounds were cell-active, capable of covalently engaging endogenous ABL kinase in K562 cells with long-residence time and few off-targets. We further validated the generality of this strategy by developing EBA-based irreversible inhibitors against EGFR (a kinase) and Mcl-1 (a non-kinase) that covalently reacted with the catalytic and noncatalytic lysine within each target.