LAM Yulin
Associate Professor
Research Fellow, Institute of Molecular and Cell Biology, 1994-1996; Research Fellow, The Scripps Research Institute, 1992-1994; Ph.D., National University of Singapore, 1992; B.Sc. (Hons), National University of Singapore, 1987.
Contact Information:
Office: S8-05-09
Tel: (65)-6516-2688
Fax: (65)-6779-1691
Email: chmlamyl@nus.edu.sg
Research
Research Interests
Our research interests include (i) bioorganic and medicinal chemistry, and (ii) green chemical synthesis. Specific foci are:
- Synthesis and biological evaluations of novel organic compounds and natural product derivatives as potential anti-cancer, anti-inflammatory and neurological agents;
- Development of recyclable reagents and catalysts for green organic transformations.
Research Highlight
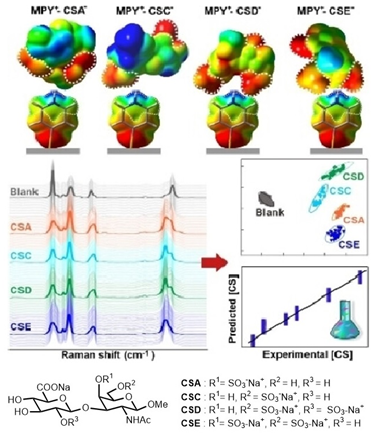
Ref: Glycosaminoglycans (GAGs) regulate many important physiological processes. A pertinent issue to address is whether GAGs encode important functional information via introduction of position specific sulfate groups in the GAG structure. However, procurement of pure, homogenous GAG motifs to probe the “sulfation code” is a challenging task due to isolation difficulty and structural complexity. To this end, we devised a divergent synthetic strategy to obtain all the 16 theoretically possible sulfation patterns in the chondroitin sulfate (CS) repeating unit; these include rare but potentially important sulfated motifs which have not been isolated earlier.
Molecular recognition of different CS analogues is achieved via surface-enhanced Raman scattering spectroscopy and by inducing charge and geometry complementarity between 4-mercaptopyridine probe and the CS isomers. By “locking” each CS in a unique complex geometry via site-specific, multidentate interactions, our platform mimics molecular docking events to enable high spectral specificity and multiplex quantification. (Angew. Chem. Int. Ed. 2023, e202309610).
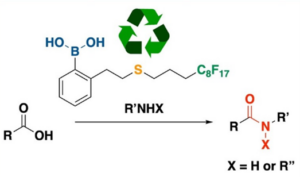
Ref: Amide bond formation reaction is one of the most frequently performed reactions in organic chemistry research laboratories, and industrially it is commonplace in polymer, chemical, agrochemical and pharmaceutical production. In view of its relevance, a plethora of strategies to generate the amide bond from alkene, alkynes, carbonyl compounds and other functional groups have been reported. Despite these developments, the direct condensation of carboxylic acids and amines in the presence of stoichiometric quantities of coupling reagents remains the most frequently employed approach to amide bond formation. However this process leads to the generation of large quantities of waste making it nonoptimal from the perspective of atom economy and cost effectiveness. To mitigate this issue, we have developed a thermally stable, fluorous sulfur-containing boronic acid which is able to efficiently promote dehydrative condensation between carboxylic acids and aminds under environmentally friendly conditions. The methodology can be applied to aliphatic, aromatic and heteroaromatic acids as wells as primary and secondary amines. N-Boc protected amino acids were also successfully coupled in good yileds with very little racemization. The catalyst could be reused four times with no significant loss of activity. (RSC Adv. 2023, 13 17420).
Teaching Contributions AY2023/2024
- CM2122 Organic Chemistry
- CM3225 Biomolecules
- CM5224 Emerging Concepts in Drug Discovery
Representative Publications
- Leong, S. X.; Kao, Y.-C.; Han, X.; Poh, Z. W.; Chen, J. R. T.; Tan, E. X.; Leong, Y. X.; Lee, Y. H.; Teo, W. X.; Yip, G. W.; Lam, Y.; Ling, X. Y., Achieving molecular recognition of structural analogues in surface-enhanced Raman scattering (SERS) spectroscopy: Inducing charge and geometry complementarity to mimic molecular docking. Angew. Chem. Int. Ed. 2023, e202309610 .
- Fridianto, K. T.; Wen, Y.-P.; Lo, L.-C.; Lam, Y., Development of Fluorous Boronic Acid Catalysts Integrated with Sulfur for Enhanced Amidation Efficiency. RSC Adv, 2023, 13, 17420-17426, DOI:10.1039/D3RA03300G.
- Fridianto, K. T.; Gunawan, G. A.; Hards, K.; Sarathy, J. P.; Cook, G. M.; Dick, T.; Go, M.-L.; Lam, Y, Alkyltriphenylphosphonium Turns Naphthoquinoneimidazoles Into Potent Membrane Depolarizers Against Mycobacteria. RSC Med Chem. 2022, 13, 1605 – 1613, DOI:10.1039/D2MD00251E.
- Fridianto, K. T.; Li, M.; Hards, K.; Negatu, D.; Cook, G.; Dick, T.; Lam, Y.; Go, M.-L., Functionalized dioxonaphthoimidazoliums: A redox cycling chemotype with potent bactericidal activities against Mycobacterium tuberculosis. J. Med. Chem. 2021, 64, 15991-16007, DOI:10.1021/acs.jmedchem.1c01383.
- Tan, Y.J.; Li, M; Gunawan, G. A.; Nyantakyi, S. A.; Dick, T.; Go, M-L.; Lam, Y., Amide-amine Replacement in Indole-2-Carboxamides Yields Potent Mycobactericidal Agents with Improved Water Solubility. ACS Med. Chem. Lett. 2021, 12, 704-712, DOI:10.1021/acsmedchemlett.0c00588.
- Gan, C.H.; Wijaya, H.; Li, L-H; Wei, C-F; Peng,Y-J; Wu, S-H; Hua, K-F; Lam, Y., H-Phosphonate Synthesis and Biological Evaluation of an Immunomodulatory Phosphoglycolipid from Thermophilic Bacteria. Org. Lett. 2020, 22, 2569-2573.
- Hsieh, C-Y; Li, L-H; Lam, Y.; Fang, Z.; Gan, C. H.; Rao, Y. K.; Chiu, H-W; Wong, W-T; Ju, T-C; Chen, F-H; Chernikov, O. V.; Liu, M-L; Hsu, C-H; Hua, K-F., Synthetic 4-hydroxy auxarconjugatin B, a novel autophagy inducer, attenuates gouty inflammation by inhibiting the NLRP3 inflammasome. Cells 2020, 9, 279-295.
- Tan, Y-J; Ali, A.; Tee, S-Y; Teo, J-T; Xi, Y.; Go, M-L; Lam, Y., Galloyl Esters of Trans-stilbenes are Inhibitors of FASN with Anticancer Activity on Non-small Cell Lung Cancer Cells. Eur. J. Med. Chem. 2019, 182, 111597-111610.
- Chng, Y. S.; Tristan, G.; Yip, G. W.; Lam, Y., Protecting-Group-Free Synthesis of Chondroitin 6-Sulfate Disaccharide and Tetrasaccharide. Org. Lett. 2019, 21, 4559-4562.
- Susanto, W.; Kong, K-H; Hua, K-F; Wu, S-H; Lam, Y., Synthesis of the Trisaccharide Repeating Unit of Capsular Polysaccharide from Klebsiella Pneumoniae. Tetrahedron Lett. 2019, 60, 288-291.

