Postdoc., Harvard Medical School, 2011; Ph.D., Harvard University, 2010; B.Sc.(Hons), National University of Singapore, 2003.
Contact Information:
Office: S9-12-01D
Tel: (65)-6516-2682
Fax: (65)-6779-1691
Email: chmchngs@nus.edu.sg
My group focuses on understanding how biological membranes are assembled in cells using bacterial outer membranes as models. Specifically, we are interested to elucidate the mechanisms of inter-membrane lipid trafficking in Gram-negative bacteria and mycobacteria and to identify protein targets in these bacteria for antibiotics discovery.
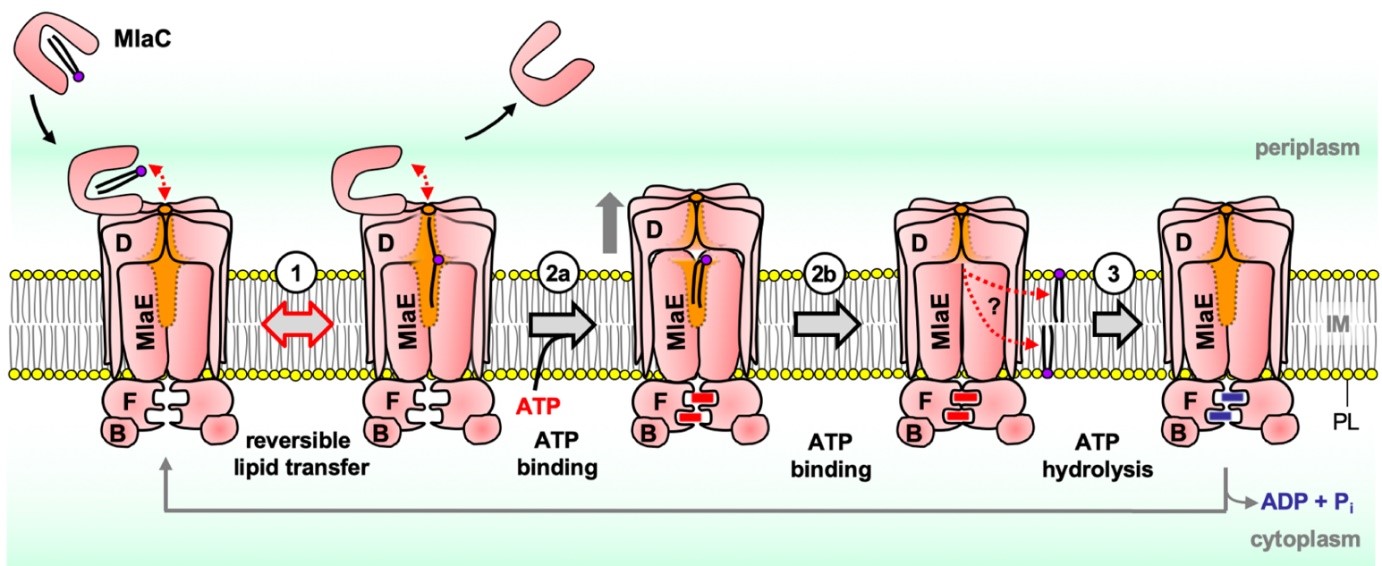
[Ref: Low W.-Y., et al. Proc. Natl. Acad. Sci. U.S.A. 2021]
The hallmark of the Gram-negative bacterial envelope is the presence of the outer membrane (OM). The OM is asymmetric, comprising lipopolysaccharides (LPS) in the outer leaflet and phospholipids (PLs) in the inner leaflet; this critical feature confers permeability barrier function against external insults, including antibiotics. To maintain OM lipid asymmetry, the OmpC-Mla system is believed to remove aberrantly localized PLs from the OM and transport them to the inner membrane (IM). Key to the system in driving lipid trafficking is the MlaFEDB ABC transporter complex in the IM, but mechanistic details, including transport directionality, remain enigmatic. Here, we develop a sensitive point-to-point in vitro lipid transfer assay that allows direct tracking of [14C]-labelled PLs between the periplasmic chaperone MlaC and MlaFEDB reconstituted into nanodiscs. We reveal that MlaC spontaneously transfers PLs to the IM transporter in an MlaD-dependent manner that can be further enhanced by coupled ATP hydrolysis. In addition, we show that MlaD is important for modulating productive coupling between ATP hydrolysis and such retrograde PL transfer. We further demonstrate that spontaneous PL transfer also occurs from MlaFEDB to MlaC, but such anterograde movement is instead abolished by ATP hydrolysis. Our work uncovers a model where PLs reversibly partition between two lipid binding sites in MlaC and MlaFEDB, and ATP binding and/or hydrolysis shift this equilibrium to ultimately drive retrograde PL transport by the OmpC-Mla system. These mechanistic insights will inform future efforts towards discovering new antibiotics against Gram-negative pathogens.