The 17th Singapore National Crystal Growing Challenge will be held on 30 May 2026 (Saturday) at the National University of Singapore. We invite students from secondary schools, international schools, junior colleges, ITE colleges, and polytechnics in Singapore to participate in this enriching scientific competition. The challenge offers students an opportunity to explore the principles and practice of crystal growth, deepening their understanding of fundamental chemical processes. Through this hands-on experience, participants will develop scientific curiosity, experimental skills, and an appreciation for the elegant chemistry underlying crystal formation. We look forward to welcoming the next generation of young scientists to engage, learn, and excel in this event.
Single Crystal of Copper(II) Sulfate Pentahydrate Copper(II) sulphate pentahydrate is one of well-known hydrate forms of this salt. The crystal structure contains pseudo-octahedral or tetragonal Cu2+ ions surrounding by six oxygen atoms of water molecules and sulphate ions, forming an infinite chain of Cu2+ and SO42- ions. This transition metal salt can be produced industrially by dissolving copper metal with hot concentrated sulfuric acid or copper oxides with dilute sulfuric acid. Bright-blue crystals of copper(II) sulphate pentahydrate can be grown from the aqueous solution of copper(II) sulphate. Owing to its redox properties of Cu(II) oxidation state, it acts as an analytical reagent to detect reducing sugars and proteins. Besides its use in electroplating industry, it is also a fungicide, herbicide, and molluscicide.
Evaluation Rubrics for Junior Category
This is just a guidance and the judges are free to select any beautiful looking and very attractive big SINGLE crystals unanimously amongst all entries.
The prizes for the winning teams:
The registration:
The Longest Single Crystal For this category, contestants are tasked to prepare a colourless, or coloured single crystal with one longest dimension. The crystal should be transparent without twining or intergrowth problem, and visible defects. Such kind of crystal should also clearly display well-defined crystal faces. If two shortlisted single crystals have the same length, the next criteria would be the actual crystal weight or size. The choices of compound for crystallisation and method of crystallisation are left to the contestants but the compound for the junior level this year must not be used. It can be an inorganic, or organic compound but the chemical name of the crystal must be stated clearly for assessment. The main objective of this category is to provide contestants with an opportunity to exercise their creativity, curiosity, individuality and scientific knowledge.
Evaluation Rubrics for Open Category
This is just a guidance and the judges are free to select any beautiful looking and very attractive big SINGLE crystals unanimously amongst all entries.
The prizes for the winning teams:
The registration:
Date: 30 May 2026 (Sat) Time: 08:45 – 14:00 Venue: General Synthetic Chemistry Teaching Laboratory, NUS Chemistry, Block S5 Level 1, Science Drive 2, Singapore 117546 [map]
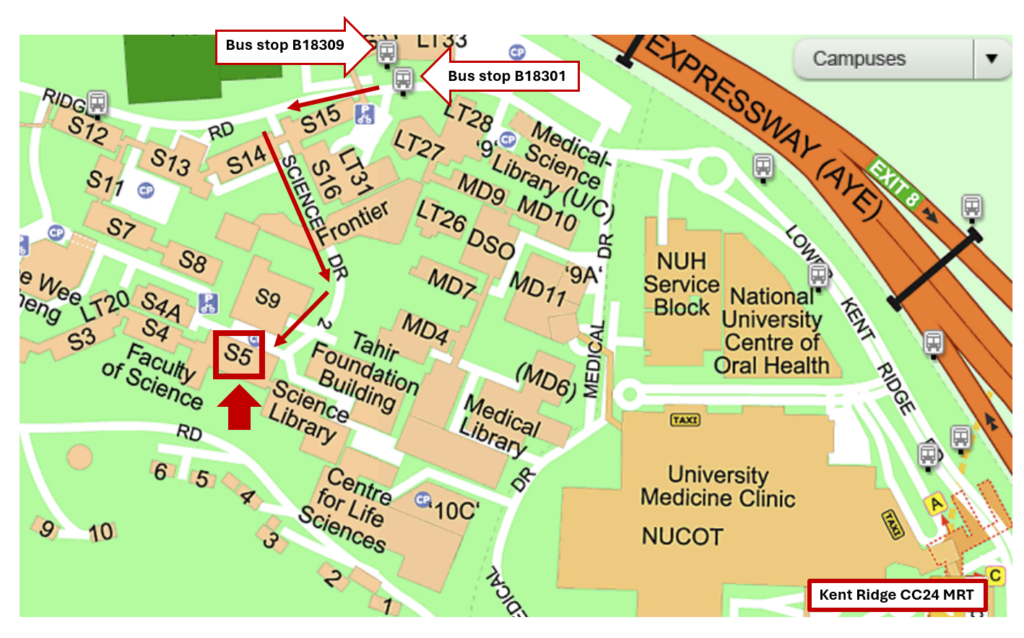
To be updated in April 2026
