Postdoctoral, University of California Berkeley/ Scripps Research Institute (USA); PhD, Purdue University (USA); B.Sc., Ohio State University (USA).
Contact Information:
Office: S9-12-01F
Tel: (65)-6516-2669
Fax: (65)-6779-1691
Email: chmyaosq@nus.edu.sg
Chemical biology and medicinal chemistry. We are interested in developing powerful strategies in Chemical Biology and Chemical Proteomics that enable organism-wide, high-throughput studies of enzymes, e.g. the so-called “catalomics”.
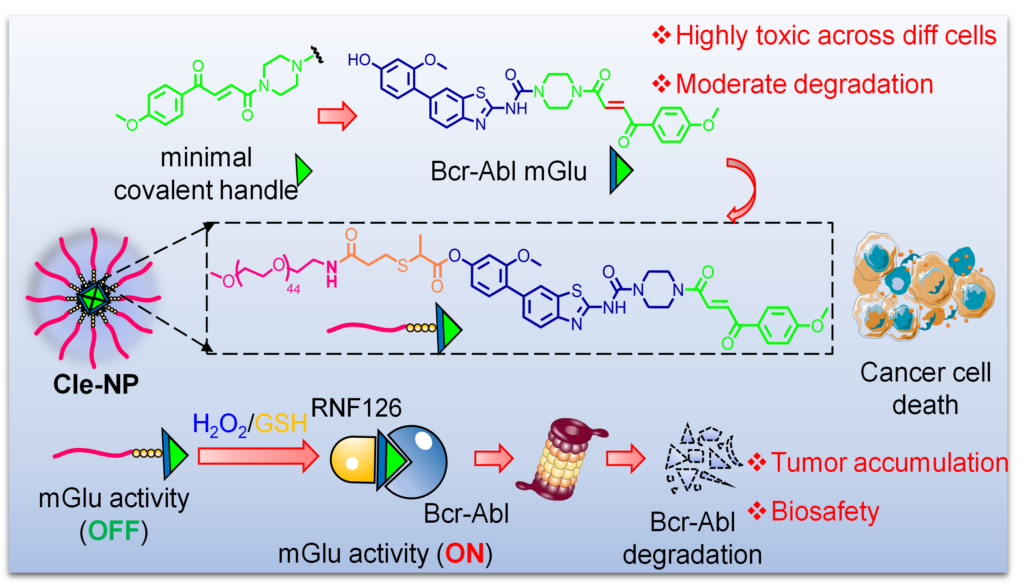
Ref. Sun, J.; Gu, M.; Peng, L.; Guo, J.; Chen, P.; Wen, Y.; Feng, F.; Chen, X.; Liu, T.; Chen, Y.; Lu, X.; Gao, L.; Yao, S. Q.; Yuan, P. A Self-Assembled Nano-Molecular Glue (Nano-mGlu) Enables GSH/H₂O₂-Triggered Targeted Protein Degradation in Cancer Therapy. J. Am. Chem. Soc. 2025, in press.
Molecular glues are promising protein-degrading agents that hold great therapeutic potential but face significant chal-lenges in rational design, effective synthesis, and precise targeting to tumor sites. In this study, we first overcame some of these limitations by introducing a fumarate-based molecular glue handle onto specific ligands of therapeutic kinases (TBK1, FGFR, and Bcr-Abl), resulting in effective degradation of these important cancer targets. Despite broad applicabil-ity of the strategy, we unexpectedly discovered potent and widespread cytotoxicity across various cell lines including noncancerous ones, rendering it less effective in cancer therapy. To address this critical issue, we next developed a self-assembled nanoparticle-based molecular glue (nano-mGlu) on the basis of one of the newly discovered Bcr-Abl-degrading molecular glue (H1-mGlu). We showed the resulting nano-mGlu (named Cle-NP) was able to release H1-mGlu in vitro, in the presence of a high concentration of GSH or H2O2 (commonly found in tumor microenvironment). Subsequent in vivo studies with a K562-xenografted mouse model indicated Cle-NP was highly effective in tumor-specific degradation of endogenous Bcr-Abl expressed in K562 cells, leading to eventual tumor regression while maintaining good biosafety profiles. With key advantages of generality in molecular glue design, targeted delivery, potent antitumor activity partially induced by target-specific degradation, and minimized collateral damage to healthy tissues, our self-assembled nano-mGlu strategy thus provides a novel approach that might hold significant prom-ises for effective and personalized cancer therapy.