KOH Ming Joo
Associate Professor, Deputy Head (Research), Dean's Chair Professor (2024-2027), National Research Foundation Investigatorship
Ph.D. and Postdoc in Organic Chemistry, Boston College, MA, 2018; B.Sc. (Hons) First Class in Chemistry & Biological Chemistry, Nanyang Technological University, 2012.
Contact Information:
Office: S9-14-01D
Tel: (65)-6601-7489
Fax: (65)-6779-1691
Email: chmkmj@nus.edu.sg
Research
Recognition and Achievements
- Asian Young Scientist Fellowship, 2025
- KYOTO Rising-Star Lectureship Award, 2025
- National Research Foundation Investigatorship, 2025
- Mitsui Chemicals Catalysis Science Award for Creative Work, 2024
- Novartis Early Career Award in Chemistry, 2023
- Asian Core Program Lectureship Award (Taiwan), 2023
- Nanyang Outstanding Young Alumni Award, NTU, 2023
- Young Researcher Award, NUS, 2023
- Asian Scientist 100, 2023
- Faculty Young Scientist Award, Faculty of Science, NUS, 2022
- Young Scientist Award, SNAS, 2022
- Asian Core Program Lectureship Award (Japan, Korea), 2022
- C&EN’s Talented 12, 2022
- Thieme Chemistry Journals Award, 2022
- TCI-SNIC Industry Award in Synthetic Chemistry, 2021
- Innovators Under 35 (TR35) Asia Pacific Award, 2021
- Excellent Young Teacher Award, 2019-2020
- Asian Core Program Lectureship Award (China, Thailand), 2019
- NUS Inauguration Grant, 2019
- President’s Assistant Professorship, 2018
Research Interests
Chemical manufacturing is one of the key pillars of the global economy and plays a crucial role in the modern human society. Chemical catalysis is an indispensable tool to promote reactions that enable access to various classes of chemicals, ranging from small-molecule medicines to polymeric materials. Despite considerable advances made in this area, much of it depends on the use of exorbitant and scarce noble metals (such as palladium) to prepare catalysts. Considering the rapidly dwindling abundance of precious metals, this approach cannot continue much longer. More industries have begun an earnest search for sustainable and cost-effective alternatives that are not subject to devastating fluctuations caused by price speculation, a well-known attribute of the precious metal market.
Furthermore, such precious metal-derived catalysts are only capable of mediating a limited range of reactions. As a result, longer synthetic sequences often have to be employed to convert a starting material to the desired target product. Unfortunately, each step in a chemical synthesis process consumes energy, resources and time, and generates waste (spent carbon-based solvents and other by-products) that has to be treated or incinerated. Based on the National Environment Agency (NEA) statistics, about 27,500,000 litres of spent solvents are collected annually in Singapore and subsequently used as supplementary fuel for toxic waste incinerators! Consequently, this leads to more CO2 and other toxic emissions that contribute to global warming, climate change, rising sea levels and other undesirable environmental problems. A lengthy and inefficient chemical synthesis process also means that manpower costs inevitably rise due to a greater demand for workers to carry out the tasks.
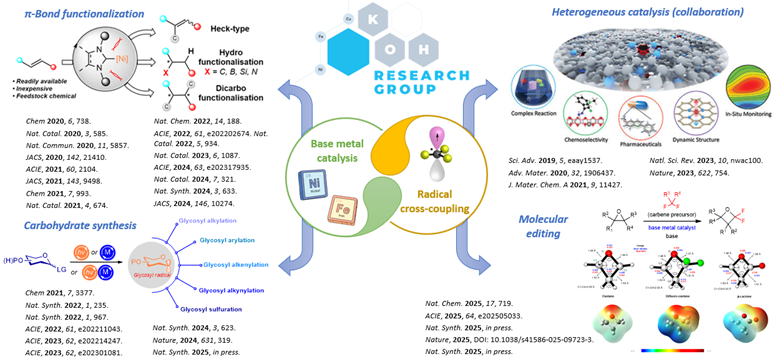
To address the aforementioned challenges, our group focuses on the research of sustainable catalysis, where we develop catalyst systems derived mainly from abundant base metals such as iron and nickel. Our efforts have led to the discovery of new multi-tasking and energy-efficient catalysts that are capable of mediating unprecedented chemical transformations, which significantly enhance the efficiency and shorten the steps required to access a target chemical product. These catalysts are employed in innovative approaches to transform cheap and abundant feedstock chemicals to value-added products with lower carbon footprint.
Research Highlight
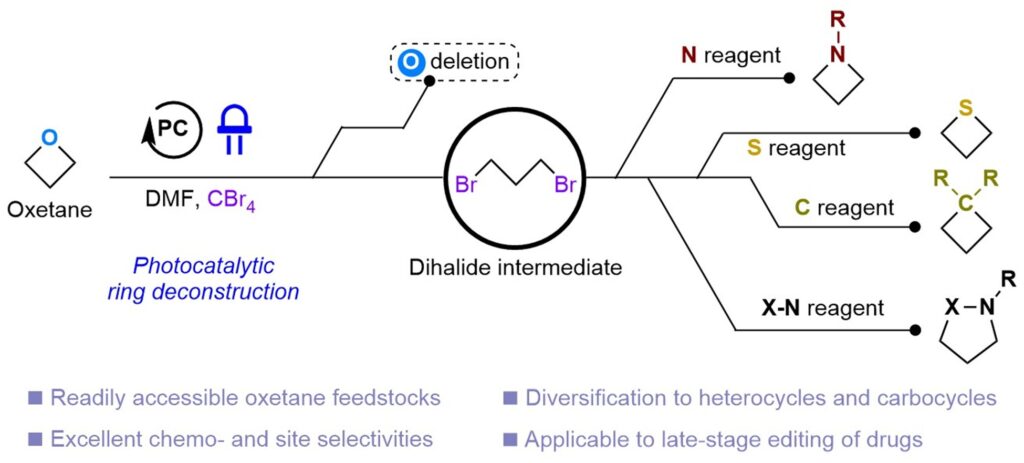
Zhang, Y.-Q.; Li, S.-H.; Zhang, X.; Koh, M. J. Photocatalytic Oxygen-Atom Transmutation of Oxetanes. Nature 2025, DOI: 10.1038/s41586-025-09723-3.
Representative Publications
- Jiang, Y.; Wei, Y.; Zhou, Q.-Y.; Sun, G.-Q; Fu, X.-P.; Levin, N.; Zhang, Y.; Liu, W.-Q.; Song, X.; Mohammed, S.; Davis, B. G.; Koh, M. J. Direct Radical Functionalization of Native Sugars. Nature 2024, 631, 319-327.
- Tan, T.-D.; Zhou, F.; Quirion, K. P.; Wang, Y.-Q.; Ng, D. Z. W.; Luo, X.; Chan, E. C. Y.; Liu, P.; Koh, M. J. Catalytic Difluorocarbene Insertion Enables Access to Fluorinated Oxetane Isosteres. Nat. Chem. 2025, 17, 719-726.
- Luo, X.; Mao, W.; Liu, C.-F.; Wang, Y.; Nie, J.; Shi, S.-L.; Ma, J.-A.; Koh, M. J. Enantioselective Synthesis of Multifunctional Alkylboronates via N-Heterocyclic Carbene-Nickel-Catalysed Carboboration of Alkenes. Nat. Synth. 2024, 3, 633-642.
- Tan, T.-D.; Serviano, J. M. I.; Luo, X.; Qian, P.-C.; Holland, P. L.; Zhang, X.; Koh, M. J. Congested C(sp3)-Rich Architectures Enabled by Iron-Catalysed Conjunctive Alkylation. Nat. Catal. 2024, 7, 321-329.
- Liu, C.-F.; Wang, Z.-C.; Luo, X.; Lu, J.; Ko, C. H. M.; Shi, S.-L.; Koh, M. J. Synthesis of Tri- and Tetrasubstituted Stereocentres by Nickel-Catalysed Enantioselective Olefin Cross-Couplings. Nat. Catal. 2022, 5, 934-942.
- Wang, Q.; Lee, B. C.; Tan, T. J.; Jiang, Y.; Ser, W. H.; Koh, M. J. Visible Light Activation Enables Desulfonylative Cross-Coupling of Glycosyl Sulfones. Nat. Synth. 2022, 1, 967-974.
- Wang, Q.; Sun, Q.; Jiang, Y.; Zhang, H.; Yu, L.; Tian, C.; Chen, G.; Koh, M. J. Iron-Catalysed Reductive Cross-Coupling of Glycosyl Radicals for the Stereoselective Synthesis of C-Glycosides. Nat. Synth. 2022, 1, 235-244.
- Wang, H.; Liu, C.-F.; Martin, R. T.; Gutierrez, O.; Koh, M. J. Directing-Group-Free Catalytic Dicarbofunctionalization of Unactivated Alkenes. Nat. Chem. 2022, 14, 188-195.
- Liu, C.-F.; Wang, H.; Martin, R. T.; Zhao, H.; Gutierrez, O.; Koh, M. J. Olefin Functionalization/Isomerization Enables Stereoselective Alkene Synthesis. Nat. Catal. 2021, 4, 674-683.
- Yu, X.; Zhao, H.; Xi, S.; Chen, Z.; Wang, X.; Wang, L.; Lin, L. Q. H.; Loh, K. P.; Koh, M. J. Site-Selective Alkene Borylation Enabled by Synergistic Hydrometallation and Borometallation. Nat. Catal. 2020, 3, 585-592.
.